Neuronal localization of presenilin-1 and association with amyloid plaques and neurofibrillary tangles in Alzheimer's disease
- PMID: 9185547
- PMCID: PMC6573321
- DOI: 10.1523/JNEUROSCI.17-13-05101.1997
Neuronal localization of presenilin-1 and association with amyloid plaques and neurofibrillary tangles in Alzheimer's disease
Abstract
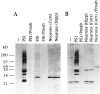
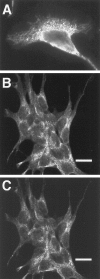
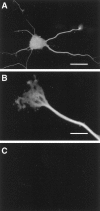
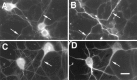
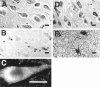
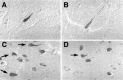
Mutations in the presenilin-1 (PS1) gene is a cause of early- onset familial Alzheimer's disease (AD). Endogenous PS1 is associated with the endoplasmic reticulum in the cell body of undifferentiated SH-SY5Y neuroblastoma cells. At early stages of neuronal differentiation in rat hippocampal culture, PS1 appears in all neuritic processes and in growth cones. In mature differentiated neurons, PS1 is concentrated in the somatodendritic compartment but is also present at lower levels in axons. A similar localization of PS1 is observed in vivo in neurons of the adult human cerebral cortex. In sporadic AD, PS1 appears in the dystrophic neurites of mature amyloid plaques and co-localizes with a subset of intraneuronal neurofibrillary tangles (NFTs). About 30% of hippocampal NFTs are labeled with a highly specific antibody to the PS1 C-terminal loop domain but not with an antibody to the PS1 N terminus. This observation is consistent with a potential association of the PS1 C-terminal fragment with NFTs, because PS1 is constitutively cleaved to N- and C-terminal fragments in neurons. These results suggest that PS1 is highly expressed and broadly distributed during early stages of neuronal differentiation, consistent with a role for PS1 in neuronal differentiation. Furthermore, the co-localization of PS1 with NFTs and plaque dystrophic neurites implicates a role for PS1 in the diverse pathological manifestations of AD.
Figures

References
-
- Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature. 1995;378:776–779. - PubMed
-
- Doan A, Thinakaran G, Borchelt DR, Slunt HH, Ratovitsky T, Podlisny M, Selkoe DJ, Seeger M, Gandy SE, Price DL, Sisodia SS. Protein topology of presenilin 1. Neuron. 1996;17:1023–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
