Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP
- PMID: 9185557
- PMCID: PMC6573299
- DOI: 10.1523/JNEUROSCI.17-13-05196.1997
Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP
Abstract
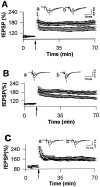
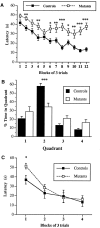
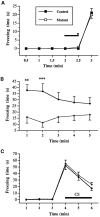
Class I metabotropic glutamate receptors (mGluRs) have been postulated to play a role in synaptic plasticity. To test the involvement of one member of this class, we have recently generated mutant mice that express no mGluR5 but normal levels of other glutamate receptors. The CNS revealed normal development of gross anatomical features. To examine synaptic functions we measured evoked field EPSPs in the hippocampal slice. Measures of presynaptic function, such as paired pulse facilitation in mutant CA1 neurons, were normal. The response of mutant CA1 neurons to low concentrations of (1S,3R)-1-amino-cyclopentane-1,3-dicarboxylic acid (ACPD) was missing, which suggests that mGluR5 may be the primary high affinity ACPD receptor in these neurons. Long-term potentiation (LTP) in mGluR5 mutants was significantly reduced in the NMDA receptor (NMDAR)-dependent pathways such as the CA1 region and dentate gyrus of the hippocampus, whereas LTP remained intact in the mossy fiber synapses on the CA3 region, an NMDAR-independent pathway. Some of the difference in CA1 LTP could lie at the level of expression, because the reduction of LTP in the mutants was no longer observed 20 min after tetanus in the presence of 2-amino-5-phosphonopentanoate. We propose that mGluR5 plays a key regulatory role in NMDAR-dependent LTP. These mutant mice were also impaired in the acquisition and use of spatial information in both the Morris water maze and contextual information in the fear-conditioning test. This is consistent with the hypothesis that LTP in the CA1 region may underlie spatial learning and memory.
Figures


References
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizumo W, Nahanishi S. Molecular characterization of a novel metabotropic glutamate receptor, mGluR5, coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. - PubMed
-
- Abeliovitch A, Paylor R, Chen C, Kim J, Wehner JM, Tonegawa S. PKCγ-mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. - PubMed
-
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. - PubMed
-
- Bach ME, Hawkins RD, Osuman M, Kendel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range and theta frequency. Cell. 1995;81:905–910. - PubMed
-
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
