The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation
- PMID: 9192664
- PMCID: PMC21257
- DOI: 10.1073/pnas.94.13.6904
The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation
Abstract
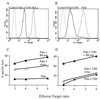
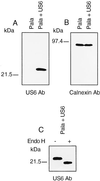
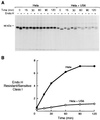
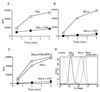
In its attempt to evade cytotoxic T cell recognition, human cytomegalovirus encodes several genes that target MHC class I molecules at different points in their assembly pathway. We show here that the human cytomegalovirus US6 gene encodes a 22-kDa glycoprotein that binds the transporter-associated with antigen processing (TAP)/class I complex and inhibits translocation of peptide from the cytosol to the endoplasmic reticulum. Major histocompatibility complex class I molecules are therefore unable to load TAP-dependent peptides, resulting in the retention of MHC class I molecules in the endoplasmic reticulum, with a consequent reduction in class I at the cell surface. Interferon-gamma treatment of US6 transfected cells overcomes this inhibition of peptide translocation and restores class I at the cell surface to wild type levels. The functional consequence of TAP inhibition is that US6 transfected cells are unable to present endogenous antigen to cytotoxic T lymphocytes and are therefore resistant to cytotoxic T lymphocyte lysis.
Figures

References
-
- Reusser P, Riddell S R, Meyers J D, Greenberg P D. Blood. 1991;78:1373–1380. - PubMed
-
- Koszinowski U H, Del Val M, Reddehase M. Curr Top Microbiol Immunol. 1990;154:189–220. - PubMed
-
- Riddell S, Wantanabe K, Goodrich J, Li C R, Agha M, Greenberg P. Science. 1992;257:238–241. - PubMed
-
- Walter E A, Greenberg P D, Gilberg M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. N Engl J Med. 1995;333:1038–1044. - PubMed
-
- Beersma M F, Bijlmakers M J, Ploegh H L. J Immunol. 1993;151:4455–4464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

