Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages
- PMID: 9192673
- PMCID: PMC21266
- DOI: 10.1073/pnas.94.13.6954
Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages
Abstract
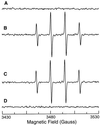
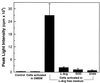
Superoxide (O-2) and nitric oxide (NO) act to kill invading microbes in phagocytes. In macrophages NO is synthesized by inducible nitric oxide synthase (iNOS, NOS 2) from L-arginine (L-Arg) and oxygen; however, O-2 was thought to be produced mainly by NADPH oxidase. Electron paramagnetic resonance (EPR) spin trapping experiments performed in murine macrophages demonstrate a novel pathway of O-2 generation. It was observed that depletion of cytosolic L-Arg triggers O-2 generation from iNOS. This iNOS-mediated O-2 generation was blocked by the NOS inhibitor N-nitro-L-arginine methyl ester or by L-Arg, but not by the noninhibitory enantiomer N-nitro-D-arginine methyl ester. In L-Arg-depleted macrophages iNOS generates both O-2 and NO that interact to form the potent oxidant peroxynitrite (ONOO-), which was detected by luminol luminescence and whose formation was blocked by superoxide dismutase, urate, or L-Arg. This iNOS-derived ONOO- resulted in nitrotyrosine formation, and this was inhibited by iNOS blockade. iNOS-mediated O-2 and ONOO- increased the antibacterial activity of macrophages. Thus, with reduced L-Arg availability iNOS produces O-2 and ONOO- that modulate macrophage function. Due to the existence of L-Arg depletion in inflammation, iNOS-mediated O-2 and ONOO- may occur and contribute to cytostatic/cytotoxic actions of macrophages.
Figures




References
-
- Bastian N R, Hibbs J B., Jr Curr Opin Immunol. 1994;6:131–139. - PubMed
-
- Nathan C, Xie Q-w. J Biol Chem. 1994;269:13725–13728. - PubMed
-
- Rosen G M, Pou S, Ramos C L, Cohen M S, Britigan B E. FASEB J. 1995;9:200–209. - PubMed
-
- Nathan C, Xie Q-w. Cell. 1994;78:915–918. - PubMed
-
- Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. J Biol Chem. 1992;267:24173–24176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

