Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons
- PMID: 9192684
- PMCID: PMC21277
- DOI: 10.1073/pnas.94.13.7018
Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons
Abstract
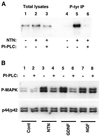
Neurturin (NTN) is a neurotrophic factor that shares homology with glial cell line-derived neurotrophic factor (GDNF). Recently, a receptor complex has been identified for GDNF that includes the Ret tyrosine kinase receptor and a glycosylphosphatidylinositol-linked protein termed "GDNFRalpha." However, differences in the phenotype of Ret and GDNF knockout animals suggest that Ret has at least one additional ligand. In this report, we demonstrate that NTN induces Ret phosphorylation in primary cultures of rat superior cervical ganglion (SCG) neurons. NTN also caused Ret phosphorylation in fibroblasts that were transfected stably with Ret and GDNFRalpha but not in cells expressing Ret alone. A glycosylphosphatidylinositol-linked protein also was important for NTN and GDNF signaling in SCG neurons; phosphatidylinositol-specific phospholipase C treatment of SCG cultures reduced the ability of NTN to phosphorylate Ret and the ability of NTN or GDNF to activate the mitogen-activated protein kinase pathway. NTN and GDNF also caused sustained activation of Ret and the mitogen-activated protein kinase pathway in SCG neurons. Finally, both NTN and GDNF activated the phosphatidylinositol 3-kinase pathway in SCG neurons, which may be important for the ability of NTN and GDNF to promote neuronal survival. These data indicate that NTN is a physiologically relevant ligand for the Ret receptor and suggest that NTN may have a critical role in the development of many neuronal populations.
Figures




References
-
- Kotzbauer P T, Lampe P A, Heuckeroth R O, Golden J P, Creedon D J, Johnson E M, Jr, Milbrandt J. Nature. 1996;384:467–470. - PubMed
-
- Trupp M, Arenas E, Fainzilber M, Nilsson A S, Sieber B A, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumäe U, Sariola H, Saarma M, Ibáñez C F. Nature (London) 1996;381:785–788. - PubMed
-
- Treanor J J, Goodman L, de Sauvage F, Stone D M, Poulsen K T, et al. Nature (London) 1996;382:80–83. - PubMed
-
- Jing S, Wen D, Yu Y, Holst P L, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis J C, Hu S, Altrock B W, Fox G M. Cell. 1996;85:1113–1124. - PubMed
-
- Durbec P, Marcos-Gutierrez C V, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V. Nature (London) 1996;381:789–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

