Glutamine synthetase protects against neuronal degeneration in injured retinal tissue
- PMID: 9192685
- PMCID: PMC21278
- DOI: 10.1073/pnas.94.13.7024
Glutamine synthetase protects against neuronal degeneration in injured retinal tissue
Abstract
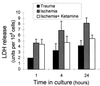
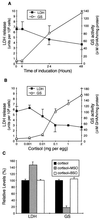
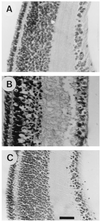
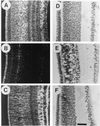
The neurotransmitter glutamate is neurotoxic when it is accumulated in a massive amount in the extracellular fluid. Excessive release of glutamate has been shown to be a major cause of neuronal degeneration after central nervous system injury. Under normal conditions, accumulation of synaptically released glutamate is prevented, at least in part, by a glial uptake system in which the glia-specific enzyme glutamine synthetase (GS) plays a key role. We postulated that glial cells cannot cope with glutamate neurotoxicity because the level of GS is not high enough to catalyze the excessive amounts of glutamate released by damaged neurons. We examined whether elevation of GS expression in glial cells protects against neuronal degeneration in injured retinal tissue. Analysis of lactate dehydrogenase efflux, DNA fragmentation, and histological sections revealed that hormonal induction of the endogenous GS gene in retinal glial cells correlates with a decline in neuronal degeneration, whereas inhibition of GS activity by methionine sulfoximine leads to increased cell death. A supply of purified GS enzyme to the culture medium of retinal explants or directly to the embryo in ovo causes a dose-dependent decline in the extent of cell death. These results show that GS is a potent neuroprotectant and that elevation of GS expression in glial cells activates an endogenous mechanism whereby neurons are protected from the deleterious effects of excess glutamate in extracellular fluid after trauma or ischemia. Our results suggest new approaches to the clinical handling of neuronal degeneration.
Figures


Similar articles
-
Glutamine synthetase enhances the clearance of extracellular glutamate by the neural retina.J Neurochem. 2002 Nov;83(3):574-80. doi: 10.1046/j.1471-4159.2002.01168.x. J Neurochem. 2002. PMID: 12390519
-
Ability of retinal Müller glial cells to protect neurons against excitotoxicity in vitro depends upon maturation and neuron-glial interactions.Glia. 1999 Feb 1;25(3):229-39. doi: 10.1002/(sici)1098-1136(19990201)25:3<229::aid-glia3>3.0.co;2-c. Glia. 1999. PMID: 9932869
-
Basic fibroblast growth factor: a potential inhibitor of glutamine synthetase expression in injured neural tissue.J Neurochem. 2001 Jun;77(6):1641-9. doi: 10.1046/j.1471-4159.2001.00390.x. J Neurochem. 2001. PMID: 11413247
-
Glutamine synthetase in brain: effect of ammonia.Neurochem Int. 2002 Aug-Sep;41(2-3):123-42. doi: 10.1016/s0197-0186(02)00033-5. Neurochem Int. 2002. PMID: 12020613 Review.
-
Mechanisms of glutamate metabolic signaling in retinal glial (Müller) cells.J Neurosci. 2000 Mar 1;20(5):1809-21. doi: 10.1523/JNEUROSCI.20-05-01809.2000. J Neurosci. 2000. PMID: 10684882 Free PMC article.
Cited by
-
Neurodegeneration as a primary change and role of neuroprotection in diabetic retinopathy.Mol Neurobiol. 2015;51(3):878-84. doi: 10.1007/s12035-014-8732-7. Epub 2014 May 15. Mol Neurobiol. 2015. PMID: 24826918 Review.
-
A splice variant acquiring an extra transcript leader region decreases the translation of glutamine synthetase gene.Biochem J. 2003 Aug 15;374(Pt 1):175-84. doi: 10.1042/BJ20030132. Biochem J. 2003. PMID: 12749766 Free PMC article.
-
The function of lactate dehydrogenase A in retinal neurons: implications to retinal degenerative diseases.PNAS Nexus. 2023 Feb 3;2(3):pgad038. doi: 10.1093/pnasnexus/pgad038. eCollection 2023 Mar. PNAS Nexus. 2023. PMID: 36896135 Free PMC article.
-
Photoreceptor Degeneration Accompanies Vascular Changes in a Zebrafish Model of Diabetic Retinopathy.Invest Ophthalmol Vis Sci. 2020 Feb 7;61(2):43. doi: 10.1167/iovs.61.2.43. Invest Ophthalmol Vis Sci. 2020. PMID: 32106290 Free PMC article.
-
Dipeptide alanine-glutamine ameliorates retinal neurodegeneration in an STZ-induced rat model.Front Pharmacol. 2024 Nov 19;15:1490443. doi: 10.3389/fphar.2024.1490443. eCollection 2024. Front Pharmacol. 2024. PMID: 39629074 Free PMC article.
References
-
- Rothman S M, Olney J W. Ann Neurol. 1986;19:105–111. - PubMed
-
- Choi D W, Rothman S M. Annu Rev Neurosci. 1990;13:171–182. - PubMed
-
- Kennedy A J, Voaden M J, Marshall J. Nature (London) 1974;252:50–52. - PubMed
-
- Moscona A A. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Vol. 2. Oxford: Permagon; 1983. pp. 111–135.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

