Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons
- PMID: 9192693
- PMCID: PMC21286
- DOI: 10.1073/pnas.94.13.7070
Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons
Erratum in
- Proc Natl Acad Sci U S A 1997 Aug 19;94(17):9511
Abstract
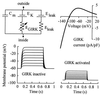
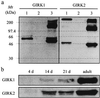
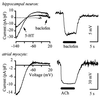
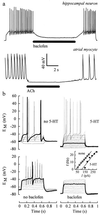
G protein-gated inward rectifier K+ channel subunits 1-4 (GIRK1-4) have been cloned from neuronal and atrial tissue and function as heterotetramers. To examine the inhibition of neuronal excitation by GIRKs, we overexpressed GIRKs in cultured hippocampal neurons from 18 day rat embryos, which normally lack or show low amounts of GIRK protein and currents. Adenoviral recombinants containing the cDNAs for GIRK1, GIRK2, GIRK4, and the serotonin 1A receptor were constructed. Typical GIRK currents could be activated by endogenous GABAB, serotonin 5-HT1A, and adenosine A1 receptors in neurons coinfected with GIRK1+2 or GIRK1+4. Under current clamp, GIRK activation increased the cell membrane conductance by 1- to 2-fold, hyperpolarized the cell by 11-14 mV, and inhibited action potential firing by increasing the threshold current for firing by 2- to 3-fold. These effects were not found in non- and mock-infected neurons, and were similar to the effects of muscarinic stimulation of native GIRK currents in atrial myocytes. Two inhibitory effects of GIRK activation, hyperpolarization and diminution of depolarizing pulses, were simulated from the experimental data. These inhibitory effects are physiologically important in the voltage range between the resting membrane potential and the potential where voltage-gated Na+ and K+ currents are activated; that is where GIRK currents are outward.
Figures
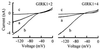
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

