The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p
- PMID: 9199167
- PMCID: PMC2137825
- DOI: 10.1083/jcb.137.7.1511
The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p
Abstract
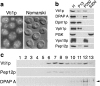
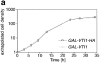
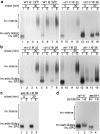
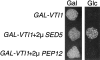
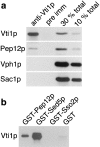
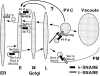
Membrane traffic in eukaryotic cells requires that specific v-SNAREs on transport vesicles interact with specific t-SNAREs on target membranes. We identified a novel Saccharomyces cerevisiae v-SNARE (Vti1p) encoded by the essential gene, VTI1. Vti1p interacts with the prevacuolar t-SNARE Pep12p to direct Golgi to prevacuolar traffic. vti1-1 mutant cells missorted and secreted the soluble vacuolar hydrolase carboxypeptidase Y (CPY) rapidly and reversibly when vti1-1 cells were shifted to the restrictive temperature. However, overexpression of Pep12p suppressed the CPY secretion defect exhibited by vti1-1 cells at 36 degrees C. Characterization of a second vti1 mutant, vti1-11, revealed that Vti1p also plays a role in membrane traffic at a cis-Golgi stage. vti1-11 mutant cells displayed a growth defect and accumulated the ER and early Golgi forms of both CPY and the secreted protein invertase at the nonpermissive temperature. Overexpression of the yeast cis-Golgi t-SNARE Sed5p suppressed the accumulation of the ER form of CPY but did not lead to CPY transport to the vacuole in vti1-11 cells. Overexpression of Sed5p allowed growth in the absence of Vti1p. In vitro binding and coimmunoprecipitation studies revealed that Vti1p interacts directly with the two t-SNAREs, Sed5p and Pep12p. These data suggest that Vti1p plays a role in cis-Golgi membrane traffic, which is essential for yeast viability, and a nonessential role in the fusion of Golgi-derived vesicles with the prevacuolar compartment. Therefore, a single v-SNARE can interact functionally with two different t-SNAREs in directing membrane traffic in yeast.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

