The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study
- PMID: 9199168
- PMCID: PMC2137822
- DOI: 10.1083/jcb.137.7.1525
The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study
Abstract
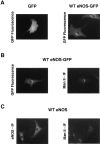
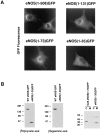
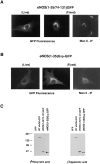
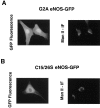
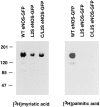
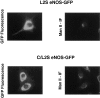
Catalytically active endothelial nitric oxide synthase (eNOS) is located on the Golgi complex and in the caveolae of endothelial cells (EC). Mislocalization of eNOS caused by mutation of the N-myristoylation or cysteine palmitoylation sites impairs production of stimulated nitric oxide (NO), suggesting that intracellular targeting is critical for optimal NO production. To investigate the molecular determinants of eNOS targeting in EC, we constructed eNOS-green fluorescent protein (GFP) chimeras to study its localization in living and fixed cells. The full-length eNOS-GFP fusion colocalized with a Golgi marker, mannosidase II, and retained catalytic activity compared to wild-type (WT) eNOS, suggesting that the GFP tag does not interfere with eNOS localization or function. Experiments with different size amino-terminal fusion partners coupled to GFP demonstrated that the first 35 amino acids of eNOS are sufficient to target GFP into the Golgi region of NIH 3T3 cells. Additionally, the unique (Gly-Leu)5 repeat located between the palmitoylation sites (Cys-15 and -26) of eNOS is necessary for its palmitoylation and thus localization, but not for N-myristoylation, membrane association, and NOS activity. The palmitoylation-deficient mutants displayed a more diffuse fluorescence pattern than did WT eNOS-GFP, but still were associated with intracellular membranes. Biochemical studies also showed that the palmitoylation-deficient mutants are associated with membranes as tightly as WT eNOS. Mutation of the N-myristoylation site Gly-2 (abolishing both N-myristoylation and palmitoylation) caused the GFP fusion protein to distribute throughout the cell as GFP alone, consistent with its primarily cytosolic nature in biochemical studies. Therefore, eNOS targets into the Golgi region of NIH 3T3 cells via the first 35 amino acids, including N-myristoylation and palmitoylation sites, and its overall membrane association requires N-myristoylation but not cysteine palmitoylation. These results suggest a novel role for fatty acylation in the specific compartmentalization of eNOS and most likely, for other dually acylated proteins, to the Golgi complex.
Figures


Similar articles
-
Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization.Biochemistry. 1996 Oct 15;35(41):13277-81. doi: 10.1021/bi961720e. Biochemistry. 1996. PMID: 8873592
-
Trafficking of endothelial nitric-oxide synthase in living cells. Quantitative evidence supporting the role of palmitoylation as a kinetic trapping mechanism limiting membrane diffusion.J Biol Chem. 1999 Aug 6;274(32):22524-31. doi: 10.1074/jbc.274.32.22524. J Biol Chem. 1999. PMID: 10428829
-
A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae.J Biol Chem. 2000 Jun 23;275(25):19416-21. doi: 10.1074/jbc.M001952200. J Biol Chem. 2000. PMID: 10787410
-
Targeting and translocation of endothelial nitric oxide synthase.Braz J Med Biol Res. 1999 Nov;32(11):1361-6. doi: 10.1590/s0100-879x1999001100006. Braz J Med Biol Res. 1999. PMID: 10559837 Review.
-
Trafficking and activation of eNOS in epithelial cells.Acta Physiol Scand. 2003 Oct;179(2):107-14. doi: 10.1046/j.1365-201X.2003.01207.x. Acta Physiol Scand. 2003. PMID: 14510773 Review.
Cited by
-
The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495 and Ser1177.Biosci Rep. 2014 Aug 6;34(4):e00129. doi: 10.1042/BSR20140079. Biosci Rep. 2014. PMID: 24993645 Free PMC article.
-
Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer.Antioxid Redox Signal. 2017 Oct 20;27(12):874-911. doi: 10.1089/ars.2017.7140. Epub 2017 Sep 8. Antioxid Redox Signal. 2017. PMID: 28712304 Free PMC article.
-
NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):17167-72. doi: 10.1073/pnas.252345399. Epub 2002 Nov 21. Proc Natl Acad Sci U S A. 2002. PMID: 12446846 Free PMC article.
-
Targeting of a short peptide derived from the cytoplasmic tail of the G1 membrane glycoprotein of Uukuniemi virus (Bunyaviridae) to the Golgi complex.J Virol. 1998 Dec;72(12):9585-96. doi: 10.1128/JVI.72.12.9585-9596.1998. J Virol. 1998. PMID: 9811692 Free PMC article.
-
Green fluorescent protein as a noninvasive intracellular pH indicator.Biophys J. 1998 Mar;74(3):1591-9. doi: 10.1016/S0006-3495(98)77870-1. Biophys J. 1998. PMID: 9512054 Free PMC article.
References
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science (Wash DC) 1994;263:802–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

