The Chlamydomonas mating type plus fertilization tubule, a prototypic cell fusion organelle: isolation, characterization, and in vitro adhesion to mating type minus gametes
- PMID: 9199169
- PMCID: PMC2137821
- DOI: 10.1083/jcb.137.7.1537
The Chlamydomonas mating type plus fertilization tubule, a prototypic cell fusion organelle: isolation, characterization, and in vitro adhesion to mating type minus gametes
Abstract
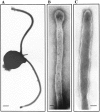
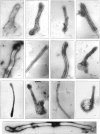
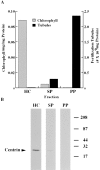
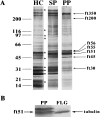
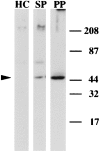
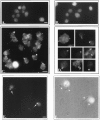
In the biflagellated alga Chlamydomonas, adhesion and fusion of the plasma membranes of gametes during fertilization occurs via an actin-filled, microvillus-like cell protrusion. Formation of this approximately 3-microm-long fusion organelle, the Chlamydomonas fertilization tubule, is induced in mating type plus (mt+) gametes during flagellar adhesion with mating type minus (mt-) gametes. Subsequent adhesion between the tip of the mt+ fertilization tubule and the apex of a mating structure on mt- gametes is followed rapidly by fusion of the plasma membranes and zygote formation. In this report, we describe the isolation and characterization of fertilization tubules from mt+ gametes activated for cell fusion. Fertilization tubules were detached by homogenization of activated mt+ gametes in an EGTA-containing buffer and purified by differential centrifugation followed by fractionation on sucrose and Percoll gradients. As determined by fluorescence microscopy of samples stained with a fluorescent probe for filamentous actin, the method yielded 2-3 x 10(6) fertilization tubules/microg protein, representing up to a 360-fold enrichment of these organelles. Examination by negative stain electron microscopy demonstrated that the purified fertilization tubules were morphologically indistinguishable from fertilization tubules on intact, activated mt+ gametes, retaining both the extracellular fringe and the internal array of actin filaments. Several proteins, including actin as well as two surface proteins identified by biotinylation studies, copurified with the fertilization tubules. Most importantly, the isolated mt+ fertilization tubules bound to the apical ends of activated mt- gametes between the two flagella, the site of the mt- mating structure; a single fertilization tubule bound per cell, binding was specific for gametes, and fertilization tubules isolated from trypsin-treated, activated mt+ gametes did not bind to activated mt- gametes.
Figures


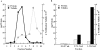




References
-
- Adair WS, Monk BC, Cohen R, Hwang C, Goodenough UW. Sexual agglutinins from the Chlamydomonasflagellar membrane: partial purification and characterization. J Biol Chem. 1982;257:4593–4602. - PubMed
-
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. - PubMed
-
- Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P, White JM. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature (Lond) 1992;356:248–252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

