IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments
- PMID: 9199170
- PMCID: PMC2137827
- DOI: 10.1083/jcb.137.7.1555
IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments
Abstract
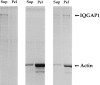
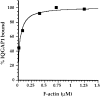
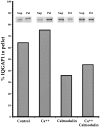
Activated forms of the GTPases, Rac and Cdc42, are known to stimulate formation of microfilament-rich lamellipodia and filopodia, respectively, but the underlying mechanisms have remained obscure. We now report the purification and characterization of a protein, IQGAP1, which is likely to mediate effects of these GTPases on microfilaments. Native IQGAP1 purified from bovine adrenal comprises two approximately 190-kD subunits per molecule plus substoichiometric calmodulin. Purified IQGAP1 bound directly to F-actin and cross-linked the actin filaments into irregular, interconnected bundles that exhibited gel-like properties. Exogenous calmodulin partially inhibited binding of IQGAP1 to F-actin, and was more effective in the absence, than in the presence of calcium. Immunofluorescence microscopy demonstrated cytochalasin D-sensitive colocalization of IQGAP1 with cortical microfilaments. These results, in conjunction with prior evidence that IQGAP1 binds directly to activated Rac and Cdc42, suggest that IQGAP1 serves as a direct molecular link between these GTPases and the actin cytoskeleton, and that the actin-binding activity of IQGAP1 is regulated by calmodulin.
Figures





References
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science (Wash DC) 1997;275:1308–1311. - PubMed
-
- Andre E, Lottspeich F, Schleicher M, Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988;263:722–727. - PubMed
-
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. - PubMed
-
- Blanchard A, Ohanian V, Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989;10:280–289. - PubMed
-
- Bloom GS, Wagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27:3409–3416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

