Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset
- PMID: 9199171
- PMCID: PMC2137823
- DOI: 10.1083/jcb.137.7.1567
Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset
Abstract
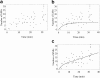
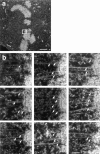
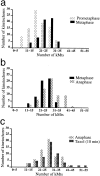
Kinetochore microtubules (kMts) are a subset of spindle microtubules that bind directly to the kinetochore to form the kinetochore fiber (K-fiber). The K-fiber in turn interacts with the kinetochore to produce chromosome motion toward the attached spindle pole. We have examined K-fiber maturation in PtK1 cells using same-cell video light microscopy/serial section EM. During congression, the kinetochore moving away from its spindle pole (i.e., the trailing kinetochore) and its leading, poleward moving sister both have variable numbers of kMts, but the trailing kinetochore always has at least twice as many kMts as the leading kinetochore. A comparison of Mt numbers on sister kinetochores of congressing chromosomes with their direction of motion, as well as distance from their associated spindle poles, reveals that the direction of motion is not determined by kMt number or total kMt length. The same result was observed for oscillating metaphase chromosomes. These data demonstrate that the tendency of a kinetochore to move poleward is not positively correlated with the kMt number. At late prometaphase, the average number of Mts on fully congressed kinetochores is 19.7 +/- 6.7 (n = 94), at late metaphase 24.3 +/- 4.9 (n = 62), and at early anaphase 27.8 +/- 6.3 (n = 65). Differences between these distributions are statistically significant. The increased kMt number during early anaphase, relative to late metaphase, reflects the increased kMt stability at anaphase onset. Treatment of late metaphase cells with 1 microM taxol inhibits anaphase onset, but produces the same kMt distribution as in early anaphase: 28.7 +/- 7. 4 (n = 54). Thus, a full complement of kMts is not sufficient to induce anaphase onset. We also measured the time course for kMt acquisition and determined an initial rate of 1.9 kMts/min. This rate accelerates up to 10-fold during the course of K-fiber maturation, suggesting an increased concentration of Mt plus ends in the vicinity of the kinetochore at late metaphase and/or cooperativity for kMt acquisition.
Figures






Similar articles
-
Dynamics of spindle microtubule organization: kinetochore fiber microtubules of plant endosperm.J Cell Biol. 1982 Feb;92(2):540-58. doi: 10.1083/jcb.92.2.540. J Cell Biol. 1982. PMID: 7061596 Free PMC article.
-
Cytochalasin J treatment significantly alters mitotic spindle microtubule organization and kinetochore structure in PtK1 cells.Cell Motil Cytoskeleton. 1997;36(2):112-24. doi: 10.1002/(SICI)1097-0169(1997)36:2<112::AID-CM2>3.0.CO;2-B. Cell Motil Cytoskeleton. 1997. PMID: 9015200
-
The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work.Mol Biol Cell. 1996 Oct;7(10):1547-58. doi: 10.1091/mbc.7.10.1547. Mol Biol Cell. 1996. PMID: 8898361 Free PMC article.
-
The KMN protein network--chief conductors of the kinetochore orchestra.J Cell Sci. 2012 Dec 15;125(Pt 24):5927-36. doi: 10.1242/jcs.093724. Epub 2013 Feb 15. J Cell Sci. 2012. PMID: 23418356 Free PMC article. Review.
-
Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle.J Cell Biol. 1994 Feb;124(3):223-33. doi: 10.1083/jcb.124.3.223. J Cell Biol. 1994. PMID: 8294508 Free PMC article. Review.
Cited by
-
Super-resolution microscopy reveals focal organization of ER-associated Y-complexes in mitosis.EMBO Rep. 2023 Sep 6;24(9):e56766. doi: 10.15252/embr.202356766. Epub 2023 Jul 20. EMBO Rep. 2023. PMID: 37469276 Free PMC article.
-
Checkpoint Proteins Bub1 and Bub3 Delay Anaphase Onset in Response to Low Tension Independent of Microtubule-Kinetochore Detachment.Cell Rep. 2019 Apr 9;27(2):416-428.e4. doi: 10.1016/j.celrep.2019.03.027. Cell Rep. 2019. PMID: 30970246 Free PMC article.
-
The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions.Nat Cell Biol. 2007 May;9(5):516-22. doi: 10.1038/ncb1576. Epub 2007 Apr 15. Nat Cell Biol. 2007. PMID: 17435749 Free PMC article.
-
Centromere tension: a divisive issue.Nat Cell Biol. 2010 Oct;12(10):919-23. doi: 10.1038/ncb1010-919. Nat Cell Biol. 2010. PMID: 20885417 Free PMC article.
-
Maloriented bivalents have metaphase positions at the spindle equator with more kinetochore microtubules to one pole than to the other.Mol Biol Cell. 2004 Dec;15(12):5346-55. doi: 10.1091/mbc.e04-06-0524. Epub 2004 Sep 22. Mol Biol Cell. 2004. PMID: 15385630 Free PMC article.
References
-
- Cassimeris L, Rieder CL, Rupp G, Salmon ED. Stability of microtubule attachment to metaphase kinetochores in PtK1cells. J Cell Sci. 1990;96:9–15. - PubMed
-
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

