Delayed development of nervous system in mice homozygous for disrupted microtubule-associated protein 1B (MAP1B) gene
- PMID: 9199175
- PMCID: PMC2137829
- DOI: 10.1083/jcb.137.7.1615
Delayed development of nervous system in mice homozygous for disrupted microtubule-associated protein 1B (MAP1B) gene
Abstract
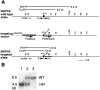
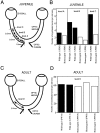
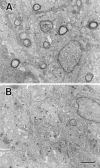
Microtubule-associated protein 1B (MAP1B), one of the microtubule-associated proteins (MAPs), is a major component of the neuronal cytoskeleton. It is expressed at high levels in immature neurons during growth of their axons, which indicates that it plays a crucial role in neuronal morphogenesis and neurite extension. To better define the role of MAP1B in vivo, we have used gene targeting to disrupt the murine MAP1B gene. Heterozygotes of our MAP1B disruption exhibit no overt abnormalities in their development and behavior, while homozygotes showed a slightly decreased brain weight and delayed nervous system development. Our data indicate that while MAP1B is not essential for survival, it is essential for normal time course development of the murine nervous system. These conclusions are very different from those of a previous MAP1B gene-targeting study (Edelmann, W., M. Zervas, P. Costello, L. Roback, I. Fischer, A. Hammarback, N. Cowan, P. Davis, B. Wainer, and R. Kucherlapati. 1996. Proc. Natl. Acad. Sci. USA. 93: 1270-1275). In this previous effort, homozygotes died before reaching 8-d embryos, while heterozygotes showed severely abnormal phenotypes in their nervous systems. Because the gene targeting event in these mice produced a gene encoding a 571-amino acid truncated product of MAP1B, it seems likely that the phenotypes seen arise from the truncated MAP1B product acting in a dominant-negative fashion, rather than a loss of MAP1B function.
Figures



Similar articles
-
Perinatal lethality of microtubule-associated protein 1B-deficient mice expressing alternative isoforms of the protein at low levels.Mol Cell Neurosci. 2000 Oct;16(4):408-21. doi: 10.1006/mcne.2000.0880. Mol Cell Neurosci. 2000. PMID: 11085878
-
MAP1B is required for axon guidance and Is involved in the development of the central and peripheral nervous system.J Cell Biol. 2000 Dec 11;151(6):1169-78. doi: 10.1083/jcb.151.6.1169. J Cell Biol. 2000. PMID: 11121433 Free PMC article.
-
Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization.J Cell Biol. 2001 Oct 1;155(1):65-76. doi: 10.1083/jcb.200106025. J Cell Biol. 2001. PMID: 11581286 Free PMC article.
-
Microtubule-associated protein 1B function during normal development, regeneration, and pathological conditions in the nervous system.J Neurobiol. 2004 Jan;58(1):48-59. doi: 10.1002/neu.10283. J Neurobiol. 2004. PMID: 14598369 Review.
-
MAP1B expression and microtubule stability in growing and regenerating axons.Microsc Res Tech. 2000 Jan 15;48(2):63-74. doi: 10.1002/(SICI)1097-0029(20000115)48:2<63::AID-JEMT2>3.0.CO;2-1. Microsc Res Tech. 2000. PMID: 10649507 Review.
Cited by
-
Microtubule-associated protein 1b is required for shaping the neural tube.Neural Dev. 2016 Jan 18;11:1. doi: 10.1186/s13064-015-0056-4. Neural Dev. 2016. PMID: 26782621 Free PMC article.
-
Human CFEOM1 mutations attenuate KIF21A autoinhibition and cause oculomotor axon stalling.Neuron. 2014 Apr 16;82(2):334-49. doi: 10.1016/j.neuron.2014.02.038. Epub 2014 Mar 20. Neuron. 2014. PMID: 24656932 Free PMC article.
-
Rab35 Functions in Axon Elongation Are Regulated by P53-Related Protein Kinase in a Mechanism That Involves Rab35 Protein Degradation and the Microtubule-Associated Protein 1B.J Neurosci. 2016 Jul 6;36(27):7298-313. doi: 10.1523/JNEUROSCI.4064-15.2016. J Neurosci. 2016. PMID: 27383602 Free PMC article.
-
Dab2ip regulates neuronal migration and neurite outgrowth in the developing neocortex.PLoS One. 2012;7(10):e46592. doi: 10.1371/journal.pone.0046592. Epub 2012 Oct 4. PLoS One. 2012. PMID: 23056358 Free PMC article.
-
Tau Gene Deletion does not Influence Axonal Regeneration and Retinal Neuron Survival in the Injured Mouse Visual System.Int J Mol Sci. 2020 Jun 8;21(11):4100. doi: 10.3390/ijms21114100. Int J Mol Sci. 2020. PMID: 32521826 Free PMC article.
References
-
- Avila J, Domínguez J, Díaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol. 1994;38:13–25. - PubMed
-
- Bates CA, Trinh N, Meyer RL. Distribution of microtubule-associated proteins (MAPs) in adult and embryonic mouse retinal explants: presence of the embryonic Map, MAP5/1B, in regenerating adult retinal axons. Dev Biol. 1993;155:533–544. - PubMed
-
- Brugg B, Reddy D, Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligonucleotides inhibits initiation of neurite outgrowth. Neuroscience. 1993;52:489–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

