ZAP-70 protein tyrosine kinase is constitutively targeted to the T cell cortex independently of its SH2 domains
- PMID: 9199177
- PMCID: PMC2137816
- DOI: 10.1083/jcb.137.7.1639
ZAP-70 protein tyrosine kinase is constitutively targeted to the T cell cortex independently of its SH2 domains
Abstract
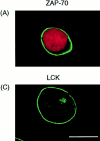
ZAP-70 is a nonreceptor protein tyrosine kinase that is essential for signaling via the T cell antigen receptor (TCR). ZAP-70 becomes phosphorylated and activated by LCK protein tyrosine kinase after interaction of its two NH2-terminal SH2 domains with tyrosine-phosphorylated subunits of the activated TCR. In this study, the localization of ZAP-70 was investigated by immunofluorescence and confocal microscopy. ZAP-70 was found to be localized to the cell cortex in a diffuse band under the plasma membrane in unstimulated T cells, and this localization was not detectably altered by TCR stimulation. Analysis of mutants indicated that ZAP-70 targeting was independent of its SH2 domains but required its active kinase domain. The specific compartmentalization of ZAP-70 suggests that it may interact with an anchoring protein in the cell cortex via its hinge or kinase domains. It is likely that the maintenance of high concentrations of ZAP-70 at the cell cortex, that only has to move a short distance to interact with phophorylated TCR subunits, facilitates rapid initiation of signaling by the TCR. In addition, as the major increase in tyrosine phosphorylation induced by the TCR also occurs at the cell cortex (Ley, S.C., M. Marsh, C.R. Bebbington, K. Proudfoot, and P. Jordan. 1994. J. Cell. Biol. 125:639-649), ZAP-70 may be localized close to its downstream targets.
Figures












Similar articles
-
Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling.J Exp Med. 1996 Feb 1;183(2):611-20. doi: 10.1084/jem.183.2.611. J Exp Med. 1996. PMID: 8627172 Free PMC article.
-
ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifs: the tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity.J Exp Med. 1995 Jan 1;181(1):375-80. doi: 10.1084/jem.181.1.375. J Exp Med. 1995. PMID: 7528772 Free PMC article.
-
Activation of Zap-70 tyrosine kinase due to a structural rearrangement induced by tyrosine phosphorylation and/or ITAM binding.Biochemistry. 2000 Mar 14;39(10):2784-91. doi: 10.1021/bi991840x. Biochemistry. 2000. PMID: 10704231
-
Why two heads are better.Structure. 1995 Oct 15;3(10):977-80. doi: 10.1016/s0969-2126(01)00232-5. Structure. 1995. PMID: 8590006 Review.
-
T cell antigen receptor signal transduction.Curr Opin Cell Biol. 1997 Apr;9(2):205-12. doi: 10.1016/s0955-0674(97)80064-6. Curr Opin Cell Biol. 1997. PMID: 9069257 Review.
Cited by
-
Involvement of crk adapter proteins in regulation of lymphoid cell functions.Immunol Res. 2003;28(2):79-91. doi: 10.1385/IR:28:2:79. Immunol Res. 2003. PMID: 14610286 Review.
-
Positive feedback between the T cell kinase Zap70 and its substrate LAT acts as a clustering-dependent signaling switch.Cell Rep. 2021 Jun 22;35(12):109280. doi: 10.1016/j.celrep.2021.109280. Cell Rep. 2021. PMID: 34161759 Free PMC article.
-
Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin.J Cell Biol. 2007 Nov 19;179(4):733-46. doi: 10.1083/jcb.200707199. J Cell Biol. 2007. PMID: 18025306 Free PMC article.
-
Genetic evidence for Shc requirement in TCR-induced c-Rel nuclear translocation and IL-2 expression.Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4544-9. doi: 10.1073/pnas.082647499. Epub 2002 Mar 26. Proc Natl Acad Sci U S A. 2002. PMID: 11917142 Free PMC article.
-
Prolactin receptor signaling: shared components with the T-cell antigen receptor in Nb2 lymphoma cells.Endocrine. 1998 Dec;9(3):313-20. doi: 10.1385/ENDO:9:3:313. Endocrine. 1998. PMID: 10221598
References
-
- Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. - PubMed
-
- Baniyash M, Garcia-Morales P, Luong E, Samelson LE, Klausner RD. The T cell antigen receptor ζ chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988;263:18225–18230. - PubMed
-
- Chan AC, Shaw AS. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Current Opin Immunol. 1996;8:394–401. - PubMed
-
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

