Dynamics of beta-catenin interactions with APC protein regulate epithelial tubulogenesis
- PMID: 9199178
- PMCID: PMC2137813
- DOI: 10.1083/jcb.137.7.1651
Dynamics of beta-catenin interactions with APC protein regulate epithelial tubulogenesis
Abstract
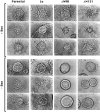
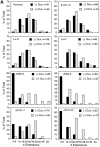
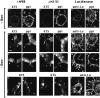
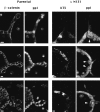
Epithelial tubulogenesis involves complex cell rearrangements that require control of both cell adhesion and migration, but the molecular mechanisms regulating these processes during tubule development are not well understood. Interactions of the cytoplasmic protein, beta-catenin, with several molecular partners have been shown to be important for cell signaling and cell-cell adhesion. To examine if beta-catenin has a role in tubulogenesis, we tested the effect of expressing NH2-terminal deleted beta-catenins in an MDCK epithelial cell model for tubulogenesis. After one day of treatment, hepatocyte growth factor/scatter factor (HGF/ SF)-stimulated MDCK cysts initiated tubulogenesis by forming many long cell extensions. Expression of NH2-terminal deleted beta-catenins inhibited formation of these cell extensions. Both DeltaN90 beta-catenin, which binds to alpha-catenin, and DeltaN131 beta-catenin, which does not bind to alpha-catenin, inhibited formation of cell extensions and tubule development, indicating that a function of beta-catenin distinct from its role in cadherin-mediated cell-cell adhesion is important for tubulogenesis. In cell extensions from parental cysts, adenomatous polyposis coli (APC) protein was localized in linear arrays and in punctate clusters at the tips of extensions. Inhibition of cell extension formation correlated with the colocalization and accumulation of NH2-terminal deleted beta-catenin in APC protein clusters and the absence of linear arrays of APC protein. Continued HGF/ SF treatment of parental cell MDCK cysts resulted in cell proliferation and reorganization of cell extensions into multicellular tubules. Similar HGF/SF treatment of cysts derived from cells expressing NH2-terminal deleted beta-catenins resulted in cells that proliferated but formed cell aggregates (polyps) within the cyst rather than tubules. Our results demonstrate an unexpected role for beta-catenin in cell migration and indicate that dynamic beta-catenin-APC protein interactions are critical for regulating cell migration during epithelial tubulogenesis.
Figures



References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. - PubMed
-
- Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127:304–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

