Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus
- PMID: 9204925
- PMCID: PMC6793833
- DOI: 10.1523/JNEUROSCI.17-14-05416.1997
Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus
Abstract
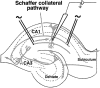
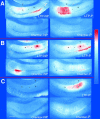
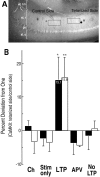
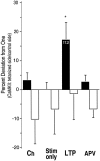
Autophosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) at threonine-286 produces Ca2+-independent kinase activity and has been proposed to be involved in induction of long-term potentiation by tetanic stimulation in the hippocampus. We have used an immunocytochemical method to visualize and quantify the pattern of autophosphorylation of CaMKII in hippocampal slices after tetanization of the Schaffer collateral pathway. Thirty minutes after tetanic stimulation, autophosphorylated CaM kinase II (P-CaMKII) is significantly increased in area CA1 both in apical dendrites and in pyramidal cell somas. In apical dendrites, this increase is accompanied by an equally significant increase in staining for nonphosphorylated CaM kinase II. Thus, the increase in P-CaMKII appears to be secondary to an increase in the total amount of CaMKII. In neuronal somas, however, the increase in P-CaMKII is not accompanied by an increase in the total amount of CaMKII. We suggest that tetanic stimulation of the Schaffer collateral pathway may induce new synthesis of CaMKII molecules in the apical dendrites, which contain mRNA encoding its alpha-subunit. In neuronal somas, however, tetanic stimulation appears to result in long-lasting increases in P-CaMKII independent of an increase in the total amount of CaMKII. Our findings are consistent with a role for autophosphorylation of CaMKII in the induction and/or maintenance of long-term potentiation, but they indicate that the effects of tetanus on the kinase and its activity are not confined to synapses and may involve induction of new synthesis of kinase in dendrites as well as increases in the level of autophosphorylated kinase.
Figures


References
-
- Abraham WC, Bear MF. Metaplasticity—the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15:1–4. - PubMed
-
- Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ. Synaptic vesicle-associated C2+/calmodulin-dependent protein kinase II is a binding-protein for synapsin I. Nature. 1992;359:417–420. - PubMed
-
- Bennett MK, Erondu NE, Kennedy MB. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983;258:12735–12744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
