Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help
- PMID: 9206998
- PMCID: PMC2198964
- DOI: 10.1084/jem.186.1.65
Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help
Abstract
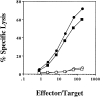
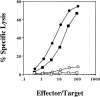
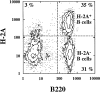
Class I-restricted presentation is usually associated with cytoplasmic degradation of cellular proteins and is often considered inaccessible to exogenous antigens. Nonetheless, certain exogenous elements can gain entry into this so-called endogenous pathway by a mechanism termed cross-presentation. This is known to be effective for class I-restricted cytotoxic T lymphocyte (CTL) cross-priming directed against a variety of exogenous tumor, viral, and minor transplantation antigens. The related effect of cross-tolerance can also effectively eliminate responses to selected self components. In both cases, this presentation appears to require the active involvement of a bone marrow-derived antigen presenting cell (APC). Here, we show that CTL induction by cross-priming with cell-associated ovalbumin requires the active involvement of CD4+ helper T cells. Importantly, this CD4+ population is only effective when both the helper and CTL determinants are recognized on the same APC. Moreover, we would argue that the cognitive nature of this event suggests that the CD4+ T cell actively modifies the APC, converting it into an effective stimulator for the successful priming of the CTL precursor.
Figures



References
-
- Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. - PubMed
-
- Gooding LR, Edwards CB. H-2 antigen requirements in the in vitro induction of SV40-specific cytotoxic T lymphocytes. J Immunol. 1980;124:1258–1262. - PubMed
-
- Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow–derived cells in presenting MHC class I–restricted tumor antigens. Science (Wash DC) 1994;264:961–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

