An interleukin 5 mutant distinguishes between two functional responses in human eosinophils
- PMID: 9207003
- PMCID: PMC2198963
- DOI: 10.1084/jem.186.1.121
An interleukin 5 mutant distinguishes between two functional responses in human eosinophils
Abstract
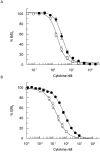
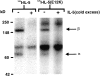
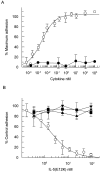
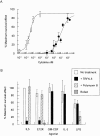
Interleukin 5 (IL-5) is the key cytokine involved in regulating the production and many of the specialized functions of mature eosinophils including priming, adhesion, and survival. We have generated a point mutant of human IL-5, IL-5 (E12K), which is devoid of agonist activity in both a TF-1 cell proliferation assay and a human eosinophil adhesion assay. However, IL-5 (E12K) is a potent and specific antagonist of both these IL-5-dependent functional responses. In both receptor binding and cross-linking studies the wild-type and IL-5 (E12K) mutant exhibit virtually identical properties. This mutant protein was unable to stimulate tyrosine phosphorylation in human eosinophils, and blocked the phosphorylation stimulated by IL-5. In contrast, IL-5 (E12K) is a full agonist in a human eosinophil survival assay, although with reduced potency compared to the wild-type protein. This IL-5 mutant enables us to clearly distinguish between two IL-5-dependent functional responses and reveals distinct mechanisms of receptor/cellular activation.
Figures


Similar articles
-
Attenuation of IL-5-mediated signal transduction, eosinophil survival, and inflammatory mediator release by a soluble human IL-5 receptor.J Immunol. 1997 Oct 15;159(8):4024-34. J Immunol. 1997. PMID: 9378992
-
[Role of interleukin-5 in immune regulation and inflammation].Nihon Rinsho. 2004 Oct;62(10):1941-51. Nihon Rinsho. 2004. PMID: 15500144 Review. Japanese.
-
Ligation of the beta2 integrin triggers activation and degranulation of human eosinophils.Am J Respir Cell Mol Biol. 1998 May;18(5):675-86. doi: 10.1165/ajrcmb.18.5.2885. Am J Respir Cell Mol Biol. 1998. PMID: 9569238
-
Functional analysis of the interleukin-5 receptor antagonist peptide, AF18748.Cytokine. 2004 Jun 21;26(6):247-54. doi: 10.1016/j.cyto.2003.10.012. Cytokine. 2004. PMID: 15183842
-
IL-5 and its receptor: which role do they play in the immune response?Int Arch Allergy Immunol. 1994 May;104(1):1-9. doi: 10.1159/000236702. Int Arch Allergy Immunol. 1994. PMID: 7950399 Review.
Cited by
-
Along the Axis between Type 1 and Type 2 Immunity; Principles Conserved in Evolution from Fish to Mammals.Biology (Basel). 2015 Nov 17;4(4):814-59. doi: 10.3390/biology4040814. Biology (Basel). 2015. PMID: 26593954 Free PMC article. Review.
-
IL-3 up-regulates and activates human eosinophil CD32 and αMβ2 integrin causing degranulation.Clin Exp Allergy. 2017 Apr;47(4):488-498. doi: 10.1111/cea.12876. Epub 2017 Jan 23. Clin Exp Allergy. 2017. PMID: 28000949 Free PMC article.
-
Antiasthmatic Effects of Herbal Complex MA and Its Fermented Product MA128.Evid Based Complement Alternat Med. 2012;2012:769508. doi: 10.1155/2012/769508. Epub 2011 Nov 15. Evid Based Complement Alternat Med. 2012. PMID: 22203879 Free PMC article.
-
Antiasthmatic effects of hesperidin, a potential Th2 cytokine antagonist, in a mouse model of allergic asthma.Mediators Inflamm. 2011;2011:485402. doi: 10.1155/2011/485402. Epub 2011 May 4. Mediators Inflamm. 2011. PMID: 21772663 Free PMC article.
-
Immunological and hematological effects of IL-5(Rα)-targeted therapy: An overview.Allergy. 2018 Oct;73(10):1979-1988. doi: 10.1111/all.13451. Epub 2018 Oct 8. Allergy. 2018. PMID: 29611207 Free PMC article. Review.
References
-
- Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of FcεRI or to calcium ionophores. Nature (Lond) 1989;339:64–67. - PubMed
-
- Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GM-CSF. Blood. 1989;73:1504–1512. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

