Transmembrane topology of a CLC chloride channel
- PMID: 9207144
- PMCID: PMC23874
- DOI: 10.1073/pnas.94.14.7633
Transmembrane topology of a CLC chloride channel
Abstract
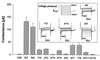
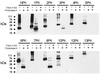
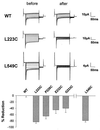
CLC chloride channels form a large and conserved gene family unrelated to other channel proteins. Knowledge of the transmembrane topology of these channels is important for understanding the effects of mutations found in human myotonia and inherited hypercalciuric kidney stone diseases and for the interpretation of structure-function studies. We now systematically study the topology of human ClC-1, a prototype CLC channel that is defective in human myotonia. Using a combination of in vitro glycosylation scanning and protease protection assays, we show that both N and C termini face the cytoplasm and demonstrate the presence of 10 (or less likely 12) transmembrane spans. Difficult regions were additionally tested by inserting cysteines and probing the effect of cysteine-modifying reagents on ClC-1 currents. The results show that D3 crosses the membrane and D4 does not, and that L549 between D11 and D12 is accessible from the outside. Further, since the modification of cysteines introduced between D11 and D12 and at the extracellular end of D3 strongly affect ClC-1 currents, these regions are suggested to be important for ion permeation.
Figures



References
-
- Greene J R, Brown N H, DiDomenico B J, Kaplan J, Eide D J. Mol Gen Genet. 1993;241:542–553. - PubMed
-
- Hechenberger M, Schwappach B, Fischer W N, Frommer W B, Jentsch T J, Steinmeyer K. J Biol Chem. 1996;271:33632–33638. - PubMed
-
- Steinmeyer K, Ortland C, Jentsch T J. Nature (London) 1991;354:301–304. - PubMed
-
- Jentsch T J, Steinmeyer K, Schwarz G. Nature (London) 1990;348:510–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

