Passive sorting in maturing granules of AtT-20 cells: the entry and exit of salivary amylase and proline-rich protein
- PMID: 9214380
- PMCID: PMC2139952
- DOI: 10.1083/jcb.138.1.45
Passive sorting in maturing granules of AtT-20 cells: the entry and exit of salivary amylase and proline-rich protein
Abstract
Previous studies have suggested that salivary amylase and proline-rich protein are sorted differently when expressed in AtT-20 cells (Castle, A.M., L.E. Stahl, and J.D. Castle. 1992. J. Biol. Chem. 267:13093- 13100; Colomer, V., K. Lal, T.C. Hoops, and M.J. Rindler. 1994.EMBO (Eur. Mol. Biol. Organ.) J. 13:3711- 3719). We now show that both exocrine proteins behave similarly and enter the regulated secretory pathway as judged by immunolocalization and secretagogue- dependent stimulation of secretion. Analysis of stimulated secretion of newly synthesized proline-rich protein, amylase, and endogenous hormones indicates that the exogenous proteins enter the granule pool with about the same efficiency as the endogenous hormones. However, in contrast to the endogenous hormones, proline-rich protein and amylase are progressively removed from the granule pool during the process of granule maturation such that only small portions remain in mature granules where they colocalize with the stored hormones. The exogenous proteins that are not stored are recovered from the incubation medium and are presumed to have undergone constitutive-like secretion. These results point to a level of sorting for regulated secretion after entry of proteins into forming granules and indicate that retention is essential for efficient storage. Consequently, the critical role of putative sorting receptors for regulated secretion may be in retention rather than in granule entry.
Figures
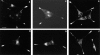


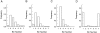
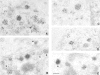

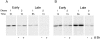
Similar articles
-
Ammonium chloride alters secretory protein sorting within the maturing exocrine storage compartment.J Biol Chem. 1989 Apr 15;264(11):6566-71. J Biol Chem. 1989. PMID: 2467913
-
Intracellular transport and secretion of salivary proteins.Crit Rev Oral Biol Med. 1998;9(1):4-22. doi: 10.1177/10454411980090010301. Crit Rev Oral Biol Med. 1998. PMID: 9488245 Review.
-
A 13-amino acid n-terminal domain of a basic proline-rich protein is necessary for storage in secretory granules and facilitates exit from the endoplasmic reticulum.J Biol Chem. 1992 Jun 25;267(18):13093-100. J Biol Chem. 1992. PMID: 1618808
-
Exocrine granule specific packaging signals are present in the polypeptide moiety of the pancreatic granule membrane protein GP2 and in amylase: implications for protein targeting to secretory granules.EMBO J. 1994 Aug 15;13(16):3711-9. doi: 10.1002/j.1460-2075.1994.tb06680.x. EMBO J. 1994. PMID: 7520866 Free PMC article.
-
Sorting and secretion of salivary proteins.Crit Rev Oral Biol Med. 1993;4(3-4):393-8. doi: 10.1177/10454411930040031901. Crit Rev Oral Biol Med. 1993. PMID: 8373994 Review.
Cited by
-
Is there structural specificity in the reversible protein aggregates that are stored in secretory granules?J Mol Neurosci. 2004;22(1-2):43-9. doi: 10.1385/JMN:22:1-2:43. J Mol Neurosci. 2004. PMID: 14742909 Review.
-
Sorting and storage during secretory granule biogenesis: looking backward and looking forward.Biochem J. 1998 Jun 15;332 ( Pt 3)(Pt 3):593-610. doi: 10.1042/bj3320593. Biochem J. 1998. PMID: 9620860 Free PMC article. Review.
-
The protozoan parasite Toxoplasma gondii targets proteins to dense granules and the vacuolar space using both conserved and unusual mechanisms.J Cell Biol. 1998 Jun 15;141(6):1323-33. doi: 10.1083/jcb.141.6.1323. J Cell Biol. 1998. PMID: 9628889 Free PMC article.
-
Efficient binding of regulated secretory protein aggregates to membrane phospholipids at acidic pH.Biochem J. 1999 Mar 1;338 ( Pt 2)(Pt 2):289-94. Biochem J. 1999. PMID: 10036223 Free PMC article.
-
Chromogranin B (secretogranin I), a neuroendocrine-regulated secretory protein, is sorted to exocrine secretory granules in transgenic mice.EMBO J. 1998 Jun 15;17(12):3277-89. doi: 10.1093/emboj/17.12.3277. EMBO J. 1998. PMID: 9628865 Free PMC article.
References
-
- Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. - PubMed
-
- Arvan R, Kuliawat R, Prabakaran D, Zavacki A, Elahi D, Wang S, Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. - PubMed
-
- Blair EA, Castle AM, Castle JD. Proteoglycan sulfation and storage parallels storage of basic secretory proteins in exocrine cells. Am J Physiol. 1991;261:C897–C905. - PubMed
-
- Castle AM, Castle JD. Novel secretory proline-rich proteoglycans from rat parotid: cloning and characterization by expression in AtT-20 cells. J Biol Chem. 1993;268:20490–20496. - PubMed

