Multiple exocytotic pathways in pancreatic beta cells
- PMID: 9214381
- PMCID: PMC2139953
- DOI: 10.1083/jcb.138.1.55
Multiple exocytotic pathways in pancreatic beta cells
Abstract
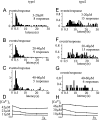
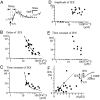
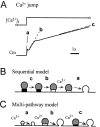
Ca2+-dependent exocytotic pathways in mouse pancreatic beta cells were investigated using both capacitance measurement and amperometric detection of vesicular contents. Serotonin was preloaded into large dense-core vesicles for the amperometry. Exocytosis was induced by rapid elevation of cytosolic Ca2+ concentrations using caged-Ca2+ compounds. Capacitance measurement revealed two major components of exocytosis, and only the slow component was accompanied by amperometric events reflecting quantal serotonin secretion. Moreover, the fast and slow exocytoses induced the two forms of endocytosis that were reported to follow the exocytoses of small-clear and large dense-core vesicles, respectively. Interestingly, we recorded two types of responses of quantal events: in the type-1 response, most quantal events occurred with a delay of 0.2 s and were rapidly exhausted with a time constant of 1.7 s, while, in the type-2 response, quantal events occurred with a delay of 2.5 s and were sustained. This suggests the existence of two pathways or modes of the exocytosis involving large dense-core vesicles. Thus, we have revealed three exocytotic pathways with divergent fusion kinetics in beta cells, which provide a new basis for the understanding of the physiology and pathology of beta cells.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

