A novel class of RanGTP binding proteins
- PMID: 9214382
- PMCID: PMC2139951
- DOI: 10.1083/jcb.138.1.65
A novel class of RanGTP binding proteins
Abstract
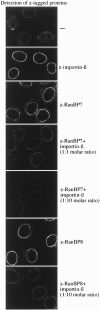
The importin-alpha/beta complex and the GTPase Ran mediate nuclear import of proteins with a classical nuclear localization signal. Although Ran has been implicated also in a variety of other processes, such as cell cycle progression, a direct function of Ran has so far only been demonstrated for importin-mediated nuclear import. We have now identified an entire class of approximately 20 potential Ran targets that share a sequence motif related to the Ran-binding site of importin-beta. We have confirmed specific RanGTP binding for some of them, namely for two novel factors, RanBP7 and RanBP8, for CAS, Pse1p, and Msn5p, and for the cell cycle regulator Cse1p from Saccharomyces cerevisiae. We have studied RanBP7 in more detail. Similar to importin-beta, it prevents the activation of Ran's GTPase by RanGAP1 and inhibits nucleotide exchange on RanGTP. RanBP7 binds directly to nuclear pore complexes where it competes for binding sites with importin-beta, transportin, and apparently also with the mediators of mRNA and U snRNA export. Furthermore, we provide evidence for a Ran-dependent transport cycle of RanBP7 and demonstrate that RanBP7 can cross the nuclear envelope rapidly and in both directions. On the basis of these results, we propose that RanBP7 might represent a nuclear transport factor that carries an as yet unknown cargo, which could apply as well for this entire class of related RanGTP-binding proteins.
Figures
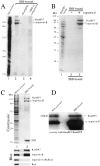
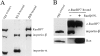
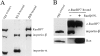





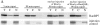
References
-
- Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. - PubMed
-
- Aitchison JD, Blobel G, Rout MP. Kap104: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science (Wash DC) 1996;274:624–627. - PubMed
-
- Altschul SF, Boguski MS, Gish W, Wootton JC. Issues in searching molecular sequence databases. Nat Genet. 1994;6:119–129. - PubMed
-
- Andrade MA, Bork P. HEAT repeats in the Huntington's disease protein. Nat Genet. 1995;11:115–116. - PubMed
-
- Becker J, Melchior F, Gerke V, Bischoff FR, Ponstingl H, Wittinghofer A. RNA1 encodes a GTPase-activating protein specific for Gsp1p, the Ran/TC4 homologue of Saccharomyces cerevisiae. . J Biol Chem. 1995;270:11860–11865. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

