Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus
- PMID: 9214387
- PMCID: PMC2139942
- DOI: 10.1083/jcb.138.1.131
Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus
Abstract
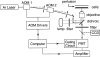
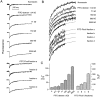
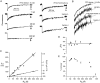
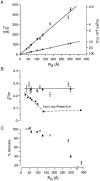
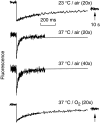
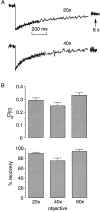
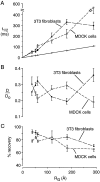
Fluorescence recovery after photobleaching (FRAP) was used to quantify the translational diffusion of microinjected FITC-dextrans and Ficolls in the cytoplasm and nucleus of MDCK epithelial cells and Swiss 3T3 fibroblasts. Absolute diffusion coefficients (D) were measured using a microsecond-resolution FRAP apparatus and solution standards. In aqueous media (viscosity 1 cP), D for the FITC-dextrans decreased from 75 to 8.4 x 10(-7) cm2/s with increasing dextran size (4-2,000 kD). D in cytoplasm relative to that in water (D/Do) was 0.26 +/- 0.01 (MDCK) and 0.27 +/- 0.01 (fibroblasts), and independent of FITC-dextran and Ficoll size (gyration radii [RG] 40-300 A). The fraction of mobile FITC-dextran molecules (fmob), determined by the extent of fluorescence recovery after spot photobleaching, was >>0.75 for RG << 200 A, but decreased to <<0.5 for RG >> 300 A. The independence of D/Do on FITC-dextran and Ficoll size does not support the concept of solute "sieving" (size-dependent diffusion) in cytoplasm. Photobleaching measurements using different spot diameters (1.5-4 micron) gave similar D/Do, indicating that microcompartments, if present, are of submicron size. Measurements of D/Do and fmob in concentrated dextran solutions, as well as in swollen and shrunken cells, suggested that the low fmob for very large macromolecules might be related to restrictions imposed by immobile obstacles (such as microcompartments) or to anomalous diffusion (such as percolation). In nucleus, D/Do was 0.25 +/- 0.02 (MDCK) and 0.27 +/- 0.03 (fibroblasts), and independent of solute size (RG 40-300 A). Our results indicate relatively free and rapid diffusion of macromolecule-sized solutes up to approximately 500 kD in cytoplasm and nucleus.
Figures



References
-
- Almeida, P.F.F., and W.L.C. Vaz. 1995. Lateral diffusion in membranes. In Handbook of Biological Physics. R. Lipowsky and E. Sackmann, editors. Elsevier Science Publishing Co., Inc., New York. 305–345.
-
- Berezney R. The nuclear matrix: a heuristic model for investigating genomic organization and function in the cell nucleus. J Cell Biochem. 1991;47:109–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

