Dendritic cells infected with poxviruses encoding MART-1/Melan A sensitize T lymphocytes in vitro
- PMID: 9220317
- PMCID: PMC2562268
- DOI: 10.1097/00002371-199707000-00004
Dendritic cells infected with poxviruses encoding MART-1/Melan A sensitize T lymphocytes in vitro
Abstract
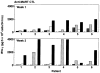
Dendritic cells (DC) are potent professional antigen-presenting cells that can activate naive T lymphocytes and initiate cellular immune responses. As adjuvants, DC may be useful in enhancing the immunogenicity of tumor antigens and mediating tumor regression. Endogenous expression of antigen by DC offers the potential advantage of allowing prolonged constitutive presentation of endogenously processed epitopes and exploitation of multiple restriction elements for the presentation of the same antigen. In this report, we show that human DC are (a) capable of infection by recombinant poxviruses encoding melanoma-associated antigen (MAA) genes and (b) capable of efficiently processing and presenting these MAA to cytotoxic T cells. In 6/6 HLA A*0201-expressing melanoma patients tested, the virally driven expression of MART-1/Melan A MAA by DC was sufficient to generate CD8+ T lymphocytes that could recognize naturally processed epitopes on tumor cells. In most cases, specific anti-MART-1 reactivity could be detected after a single stimulation. Analysis of epitope dominance revealed that the amino acid sequence recognized by these cytotoxic T lymphocytes (CTL) corresponded to the MART-1(27-35) residues previously shown to be most commonly recognized by cytotoxic T lymphocytes expanded from metastatic melanoma lesions. These data show that the virally driven expression of MAA by DC can be exploited for the efficient induction of clinically relevant cytotoxic T-cell responses. This has clinical implications for active immunization therapy, and currently vaccine trials have been proposed for patients with metastatic melanoma.
Figures


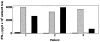

References
-
- van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. - PubMed
-
- Kawakami Y, Eliyahu S, Jennings C, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–8. - PubMed
-
- Wolfel T, Van Pel A, Brichard V, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–64. - PubMed
-
- Rivoltini L, Kawakami Y, Sakaguchi K, et al. Induction of tumor reactive CTL from peripheral blood and tumor infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J Immunol. 1995;154:2257–65. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
