Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells
- PMID: 9221753
- PMCID: PMC2198972
- DOI: 10.1084/jem.186.2.239
Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells
Abstract
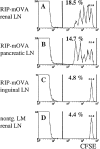
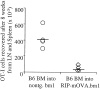
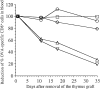
In this report, we show that cross-presentation of self-antigens can lead to the peripheral deletion of autoreactive CD8(+) T cells. We had previously shown that transfer of ovalbumin (OVA)-specific CD8(+) T cells (OT-I cells) into rat insulin promoter-membrane-bound form of OVA transgenic mice, which express the model autoantigen OVA in the proximal tubular cells of the kidneys, the beta cells of the pancreas, the thymus, and the testis of male mice, led to the activation of OT-I cells in the draining lymph nodes. This was due to class I-restricted cross-presentation of exogenous OVA on a bone marrow-derived antigen presenting cell (APC) population. Here, we show that adoptively transferred or thymically derived OT-I cells activated by cross-presentation are deleted from the peripheral pool of recirculating lymphocytes. Such deletion only required antigen recognition on a bone marrow-derived population, suggesting that cells of the professional APC class may be tolerogenic under these circumstances. Our results provide a mechanism by which the immune system can induce CD8(+) T cell tolerance to autoantigens that are expressed outside the recirculation pathway of naive T cells.
Figures



References
-
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. - PubMed
-
- Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. - PubMed
-
- Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. - PubMed
-
- Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

