Assembly and regulation of the CD40 receptor complex in human B cells
- PMID: 9221764
- PMCID: PMC2198982
- DOI: 10.1084/jem.186.2.337
Assembly and regulation of the CD40 receptor complex in human B cells
Abstract
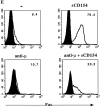
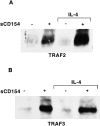
CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily. Studies with human B cells show that the binding of CD154 (gp39, CD40L) to CD40 recruits TNF receptor- associated factor 2 (TRAF2) and TRAF3 to the receptor complex, induces the downregulation of the nonreceptor-associated TRAFs in the cell and induces an increased expression of Fas on the cell surface. Combined signaling through the interluekin 4 receptor and CD40 induces an increased expression of Fas with a commensurate increase in the level of TRAF2, but not TRAF3, that is recruited to the receptor complex. In contrast, engagement of the membrane immunoglobulin and CD40 limits Fas upregulation and reduces the recruitment of TRAF2, relative to TRAF3, to the CD40 receptor complex. These studies show that the TRAF composition of the CD40 receptor complex can be altered by signals that influence B cell differentiation.
Figures




References
-
- Foy T, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. - PubMed
-
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. - PubMed
-
- Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature (Lond) 1995;378:617–620. - PubMed
-
- Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. - PubMed
-
- Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

