Calcium signaling in transgenic mice overexpressing cardiac Na(+)-Ca2+ exchanger
- PMID: 9222898
- PMCID: PMC2217045
- DOI: 10.1085/jgp.109.6.717
Calcium signaling in transgenic mice overexpressing cardiac Na(+)-Ca2+ exchanger
Abstract
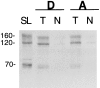
We have produced transgenic mice which overexpress cardiac Na(+)-Ca2+ exchange activity. Overexpression has been assessed by Western blot, Northern blot, and immunofluorescence. Functional overexpression was analyzed using membrane vesicles and isolated ventricular myocytes. In whole cell clamped myocytes dialyzed with 0.1-0.2 mM Fura-2, the magnitude of ICa and Ca2+i-transient triggered by ICa or caffeine were not significantly different in transgenic vs. control myocytes. In transgenic myocytes, activation of ICa, however, was followed by a large slowly inactivating transient inward current representing INa-Ca. This current depended on Ca2+ release as it was abolished when sarcoplasmic reticulum (SR) Ca2+ was depleted using thapsigargin. Cai-transients triggered by rapid application of 5 mM caffeine, even though equivalent in control and transgenic myocytes, activated larger INa-Ca (approximately 5 pA/pF at -90 mV) in transgenic vs. control myocytes (1.5 pA/pF). The decay rate of caffeine-induced Ca2+i-transient and INa-Ca was 2.5 times faster in transgenic than in control myocytes. 5 mM Ni2+ was equally effective in blocking INa-Ca in control or transgenic myocytes. In 9 out of 26 transgenic myocytes, but none of the controls, Ca2+ influx via the exchanger measured at +80 mV caused a slow rise in [Ca2+]i triggering rapid release of Ca2+ from the SR, SR Ca2+ release triggered by the exchanger at such potentials was accompanied by activation of transient current in the inward direction. In 2 mM Fura-2-dialyzed transgenic myocytes caffeine-triggered Cai-transients failed to activate INa-Ca even though the kinetics of inactivation of ICa slowed significantly in caffeine-treated myocytes. In 0.1 mM Fura-2-dialyzed transgenic myocytes 100 microM Cd2+ effectively blocked ICa and suppressed Cai-transients at -10 or +50 mV. Our data suggests that in myocytes overexpressing the exchanger, the content of intracellular Ca2+ pools and the signaling of its release by the Ca2+ channel vis-à-vis the Na(+)-Ca2+ exchanger were not significantly altered despite an up to ninefold increase in the exchanger activity. We conclude that the exchanger remains functionally excluded from the Ca2+ microdomains surrounding the DHP/ryanodine receptor complex.
Figures









References
-
- Callewaert G. Excitation-contraction coupling in mammalian cardiac cells. Cardiovasc Res. 1992;26:923–932. - PubMed
-
- Callewaert G, Cleemann L, Morad M. Caffeine-induced Ca2+-release activates Ca2+ extrusion via Na+-Ca2+exchanger in cardiac myocytes. Am J Physiol. 1989;257:C147–152. - PubMed
-
- Chen F, Mottino G, Klitzner TS, Philipson KD, Frank JS. Distribution of the Na+/Ca2+exchange protein in developing rabbit myocytes. Am J Physiol. 1995;268:C1126–C1132. - PubMed
-
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

