Alpha-tocopheryl hydroquinone is an efficient multifunctional inhibitor of radical-initiated oxidation of low density lipoprotein lipids
- PMID: 9223282
- PMCID: PMC21524
- DOI: 10.1073/pnas.94.15.7885
Alpha-tocopheryl hydroquinone is an efficient multifunctional inhibitor of radical-initiated oxidation of low density lipoprotein lipids
Abstract
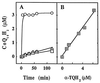
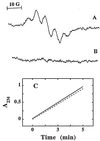
As the oxidation of low density lipoprotein (LDL) lipids may be a key event in atherogenesis, there is interest in antioxidants as potential anti-atherogenic compounds. Here we report that alpha-tocopheryl hydroquinone (alpha-TQH2) strongly inhibited or completely prevented the (per)oxidation of ubiquinol-10 (CoQ10H2), alpha-tocopherol (alpha-TOH), and both surface and core lipids in LDL exposed to either aqueous or lipophilic peroxyl radicals, Cu2+, soybean lipoxygenase, or the transition metal-containing Ham's F-10 medium in the absence or presence of human monocyte-derived macrophages. The antioxidant activity of alpha-TQH2 was superior to that of several other lipophilic hydroquinones, including endogenous CoQ10H2, which is regarded as LDL's first line of antioxidant defence. At least three independent activities contributed to the antioxidant action of alpha-TQH2. First, alpha-TQH2 readily associated with LDL and instantaneously reduced the lipoprotein's ubiquinone-10 to CoQ10H2, thereby maintaining this antioxidant in its active form. Second, alpha-TQH2 directly intercepted aqueous peroxyl radicals, as indicated by the increased rate of its consumption with increasing rates of radical production, independent of LDL's content of CoQ10H2 and alpha-TOH. Third, alpha-TQH2 rapidly quenched alpha-tocopheroxyl radical in oxidizing LDL, as demonstrated directly by electron paramagnetic resonance spectroscopy. Similar antioxidant activities were also seen when alpha-TQH2 was added to high-density lipoprotein or the protein-free Intralipid, indicating that the potent antioxidant activity of alpha-TQH2 was neither lipoprotein specific nor dependent on proteins. These results suggest that alpha-TQH2 is a candidate for a therapeutic lipid-soluble antioxidant. As alpha-tocopherylquinone is formed in vivo at sites of oxidative stress, including human atherosclerotic plaque, and biological systems exist that reduce the quinone to the hydroquinone, our results also suggest that alpha-TQH2 could be a previously unrecognized natural antioxidant.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

