Acetylcholinesterase-transgenic mice display embryonic modulations in spinal cord choline acetyltransferase and neurexin Ibeta gene expression followed by late-onset neuromotor deterioration
- PMID: 9223334
- PMCID: PMC21576
- DOI: 10.1073/pnas.94.15.8173
Acetylcholinesterase-transgenic mice display embryonic modulations in spinal cord choline acetyltransferase and neurexin Ibeta gene expression followed by late-onset neuromotor deterioration
Abstract
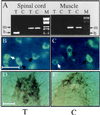
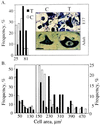
To explore the possibility that overproduction of neuronal acetylcholinesterase (AChE) confers changes in both cholinergic and morphogenic intercellular interactions, we studied developmental responses to neuronal AChE overexpression in motoneurons and neuromuscular junctions of AChE-transgenic mice. Perikarya of spinal cord motoneurons were consistently enlarged from embryonic through adult stages in AChE-transgenic mice. Atypical motoneuron development was accompanied by premature enhancement in the embryonic spinal cord expression of choline acetyltransferase mRNA, encoding the acetylcholine-synthesizing enzyme choline acetyltransferase. In contrast, the mRNA encoding for neurexin-Ibeta, the heterophilic ligand of the AChE-homologous neuronal cell surface protein neuroligin, was drastically lower in embryonic transgenic spinal cord than in controls. Postnatal cessation of these dual transcriptional responses was followed by late-onset deterioration in neuromotor performance that was associated with gross aberrations in neuromuscular ultrastructure and with pronounced amyotrophy. These findings demonstrate embryonic feedback mechanisms to neuronal AChE overexpression that are attributable to both cholinergic and cell-cell interaction pathways, suggesting that embryonic neurexin Ibeta expression is concerted in vivo with AChE levels and indicating that postnatal changes in neuronal AChE-associated proteins may be involved in late-onset neuromotor pathologies.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

