Interaction between amyloid precursor protein and presenilins in mammalian cells: implications for the pathogenesis of Alzheimer disease
- PMID: 9223340
- PMCID: PMC21582
- DOI: 10.1073/pnas.94.15.8208
Interaction between amyloid precursor protein and presenilins in mammalian cells: implications for the pathogenesis of Alzheimer disease
Abstract
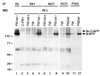
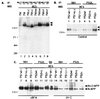
Mutations in the presenilin 1 (PS1) and presenilin 2 (PS2) genes increase the production of the highly amyloidogenic 42-residue form of amyloid beta-protein (Abeta42) in a variety of cell lines and transgenic mice. To elucidate the molecular mechanism of this effect, wild-type (wt) or mutant PS1 and PS2 genes were stably transfected into Chinese hamster ovary cells expressing endogenous or transfected beta-amyloid precursor protein (APP). By immunoprecipitation/Western blot analysis, APP was consistently found to coimmunoprecipitate with PS1 or PS2 proteins. Several distinct PS1, PS2, or APP antibodies precipitated PS-APP complexes that were detectable by blotting with either APP or PS antibodies. Importantly, complex formation could be detected at endogenous protein levels in nontransfected cells. In various Chinese hamster ovary cell lines, the amounts of APP coprecipitated by PS antibodies were proportional to the expression levels of both APP and PS. APP-PS complexes also were recovered from human 293 and HS683 cells. Full maturation of APP was not required for the interaction; most APP molecules complexed with PS were solely N-glycosylated. Treatment of cells with brefeldin A or incubation at 20 degrees C did not block complex formation, suggesting that the association between APP and PS occurs in part in the endoplasmic reticulum. Complex formation was detected for both wt and mutant PS and APP proteins. Deletion of the APP C-terminal domain did not abrogate complex formation, suggesting that the interaction does not occur in the cytoplasmic domains of the proteins. Our results demonstrate that wt and mutant PS1 and PS2 proteins form complexes with APP in living cells, strongly supporting the hypothesis that mutant PS interacts with APP in a way that enhances the intramembranous proteolysis of the latter by a gamma-secretase cleaving at Abeta42.
Figures

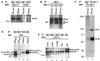
References
-
- Selkoe D J. Science. 1997;275:630–631. - PubMed
-
- Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, et al. Nature (London) 1995;375:754–760.
-
- Rogaev E I, Sherrington R, Rogaeva E A, Levesque G, Ikeda M, et al. Nature (London) 1995;376:775–778. - PubMed
-
- Levy-Lahad E, Wasco W, Poorkaj P, Romano D M, Oshima J, Pettingell H, Yu C, Jondro P D, Schmidt S D, Wang K, Crowley A C, Fu Y-H, Guenette S Y, Galas D, Nemens E, Wijsman E M, Bird T D, Schellenberg G D, Tanzi R E. Science. 1995;269:973–977. - PubMed
-
- Borchelt D, Thinakaran G, Eckman C, Lee M, Davenport F, Ratovitsky T, Prada C, Kim G, Seekins S, Yager D, Slunt H, Wang R, Seeger M, Levey A, Gandy S, Copeland N, Jenkins N, Price D, Younkin S, Sisodia S. Neuron. 1996;17:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

