Opioid receptors from a lower vertebrate (Catostomus commersoni): sequence, pharmacology, coupling to a G-protein-gated inward-rectifying potassium channel (GIRK1), and evolution
- PMID: 9223341
- PMCID: PMC21583
- DOI: 10.1073/pnas.94.15.8214
Opioid receptors from a lower vertebrate (Catostomus commersoni): sequence, pharmacology, coupling to a G-protein-gated inward-rectifying potassium channel (GIRK1), and evolution
Abstract
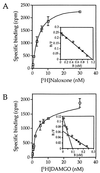
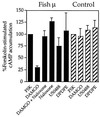
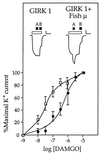
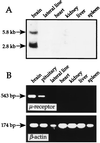
The molecular evolution of the opioid receptor family has been studied by isolating cDNAs that encode six distinct opioid receptor-like proteins from a lower vertebrate, the teleost fish Catostomus commersoni. One of these, which has been obtained in full-length form, encodes a 383-amino acid protein that exhibits greatest sequence similarity to mammalian mu-opioid receptors; the corresponding gene is expressed predominantly in brain and pituitary. Transfection of the teleost cDNA into HEK 293 cells resulted in the appearance of a receptor having high affinity for the mu-selective agonist [D-Ala2, MePhe4-Gly-ol5]enkephalin (DAMGO) (Kd = 0.63 +/- 0.15 nM) and for the nonselective antagonist naloxone (Kd = 3.1 +/- 1.3 nM). The receptor had negligible affinity for U50488 and [D-Pen2, D-Pen5]enkephalin (DPDPE), which are kappa- and delta-opioid receptor selective agonists, respectively. Stimulation of transfected cells with 1 microM DAMGO lowered forskolin-induced cAMP levels, an effect that could be reversed by naloxone. Experiments in Xenopus oocytes have demonstrated that the fish opioid receptor can, in an agonist-dependent fashion, activate a coexpressed mouse G-protein-gated inward-rectifying potassium channel (GIRK1). The identification of six distinct fish opioid receptor-like proteins suggests that additional mammalian opioid receptors remain to be identified at the molecular level. Furthermore, our data indicate that the mu-opioid receptor arose very early in evolution, perhaps before the appearance of vertebrates, and that the pharmacological and functional properties of this receptor have been conserved over a period of approximately 400 million years implying that it fulfills an important physiological role.
Figures



Similar articles
-
Functional couplings of the delta- and the kappa-opioid receptors with the G-protein-activated K+ channel.Biochem Biophys Res Commun. 1995 Mar 8;208(1):302-8. doi: 10.1006/bbrc.1995.1338. Biochem Biophys Res Commun. 1995. PMID: 7887943
-
Effects of clozapine on the delta- and kappa-opioid receptors and the G-protein-activated K+ (GIRK) channel expressed in Xenopus oocytes.Br J Pharmacol. 1998 Feb;123(3):421-6. doi: 10.1038/sj.bjp.0701621. Br J Pharmacol. 1998. PMID: 9504382 Free PMC article.
-
kappa-Opioid receptor activates an inwardly rectifying K+ channel by a G protein-linked mechanism: coexpression in Xenopus oocytes.Mol Pharmacol. 1995 May;47(5):1035-40. Mol Pharmacol. 1995. PMID: 7746270
-
Comparison of the three mouse G-protein-activated K+ (GIRK) channels and functional couplings of the opioid receptors with the GIRK1 channel.Ann N Y Acad Sci. 1996 Oct 31;801:95-109. doi: 10.1111/j.1749-6632.1996.tb17434.x. Ann N Y Acad Sci. 1996. PMID: 8959026 Review. No abstract available.
-
Opioid research in amphibians: an alternative pain model yielding insights on the evolution of opioid receptors.Brain Res Brain Res Rev. 2004 Oct;46(2):204-15. doi: 10.1016/j.brainresrev.2004.07.003. Brain Res Brain Res Rev. 2004. PMID: 15464208 Free PMC article. Review.
Cited by
-
Spotlight on Nociceptin/Orphanin FQ Receptor in the Treatment of Pain.Molecules. 2022 Jan 18;27(3):595. doi: 10.3390/molecules27030595. Molecules. 2022. PMID: 35163856 Free PMC article. Review.
-
Mu opioids and their receptors: evolution of a concept.Pharmacol Rev. 2013 Sep 27;65(4):1257-317. doi: 10.1124/pr.112.007138. Print 2013. Pharmacol Rev. 2013. PMID: 24076545 Free PMC article. Review.
-
Single Amino Acid Variation Underlies Species-Specific Sensitivity to Amphibian Skin-Derived Opioid-like Peptides.Chem Biol. 2015 Jun 18;22(6):764-75. doi: 10.1016/j.chembiol.2015.05.012. Chem Biol. 2015. PMID: 26091169 Free PMC article.
-
A pharmacological comparison of the cloned frog and human mu opioid receptors reveals differences in opioid affinity and function.Eur J Pharmacol. 2008 Dec 3;599(1-3):36-43. doi: 10.1016/j.ejphar.2008.09.043. Epub 2008 Oct 9. Eur J Pharmacol. 2008. PMID: 18930720 Free PMC article.
-
Urotensin I-CRF-Urocortins: a mermaid's tail.Gen Comp Endocrinol. 2009 Oct;164(1):7-14. doi: 10.1016/j.ygcen.2009.04.024. Epub 2009 May 4. Gen Comp Endocrinol. 2009. PMID: 19409389 Free PMC article.
References
-
- Goldstein A. Trends Pharmacol Sci. 1987;8:456–459.
-
- Loh H H, Smith A P. Annu Rev Pharmacol Toxicol. 1990;30:123–147. - PubMed
-
- Barnard E A, Simon J. In: Neurotransmitter Receptors. Hucho F, editor. Amsterdam: Elsevier; 1993. pp. 297–323.
-
- Evans C J, Keith D E, Jr, Morrison H, Magendzo K, Edwards R H. Science. 1992;258:1952–1955. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

