Thrombin stimulates glucose transport in human platelets via the translocation of the glucose transporter GLUT-3 from alpha-granules to the cell surface
- PMID: 9230074
- PMCID: PMC2138201
- DOI: 10.1083/jcb.138.2.323
Thrombin stimulates glucose transport in human platelets via the translocation of the glucose transporter GLUT-3 from alpha-granules to the cell surface
Abstract
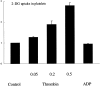
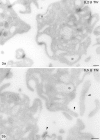
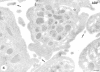
Increased energy metabolism in the circulating blood platelet plays an essential role in platelet plug formation and clot retraction. This increased energy consumption is mainly due to enhanced anaerobic consumption of glucose via the glycolytic pathway. The aim of the present study was to determine the role of glucose transport as a potential rate-limiting step for human platelet glucose metabolism. We measured in isolated platelet preparations the effect of thrombin and ADP activation, on glucose transport (2-deoxyglucose uptake), and the cellular distribution of the platelet glucose transporter (GLUT), GLUT-3. Thrombin (0.5 U/ml) caused a pronounced shape change and secretion of most alpha-granules within 10 min. During that time glucose transport increased approximately threefold, concomitant with a similar increase in expression of GLUT-3 on the plasma membrane as observed by immunocytochemistry. A major shift in GLUT-3 labeling was observed from the alpha-granule membranes in resting platelets to the plasma membrane after thrombin treatment. ADP induced shape change but no significant alpha-granule secretion. Accordingly, ADP-treated platelets showed no increased glucose transport and no increased GLUT-3 labeling on the plasma membrane. These studies suggest that, in human blood platelets, increased energy metabolism may be precisely coupled to the platelet activation response by means of the translocation of GLUT-3 by regulated secretion of alpha-granules. Observations in megakaryocytes and platelets freshly fixed from blood confirmed the predominant GLUT-3 localization in alpha-granules in the isolated cells, except that even less GLUT-3 is present at the plasma membrane in the circulating cells (approximately 15%), indicating that glucose uptake may be upregulated five to six times during in vivo activation of platelets.
Figures

References
-
- Akkerman JWN. Regulation of carbohydrate metabolism in platelets. A Review. Thromb Haemostasis. 1978;39:712–724. - PubMed
-
- Akkerman, J.W.N. 1987. Carbohydrate metabolism. In Platelet Responses and Metabolism. H. Holmsen, editor. CRC Press, Inc., Boca Raton, FL. 189–213.
-
- Behnke O. Microtubules and microfilaments. Triangle. 1974;13:7–15. - PubMed
-
- Bettex-Galland M, Luscher EF. Extraction of an actomyosin-like protein from human thrombocytes. Nature (Lond) 1959;184:276–277. - PubMed

