Regulation of actin polymerization in cell-free systems by GTPgammaS and Cdc42
- PMID: 9230078
- PMCID: PMC2138194
- DOI: 10.1083/jcb.138.2.363
Regulation of actin polymerization in cell-free systems by GTPgammaS and Cdc42
Abstract
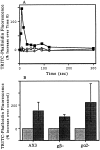
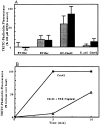
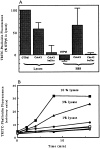
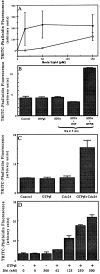
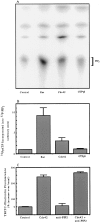
We have established a cell-free system to investigate pathways that regulate actin polymerization. Addition of GTPgammaS to lysates of polymorphonuclear leukocytes (PMNs) or Dictyostelium discoideum amoeba induced formation of filamentous actin. The GTPgammaS appeared to act via a small G-protein, since it was active in lysates ofD. discoideum mutants missing either the alpha2- or beta-subunit of the heterotrimeric G-protein required for chemoattractant-induced actin polymerization in living cells. Furthermore, recombinant Cdc42, but not Rho or Rac, induced polymerization in the cell-free system. The Cdc42-induced increase in filamentous actin required GTPgammaS binding and was inhibited by a fragment of the enzyme PAK1 that binds Cdc42. In a high speed supernatant, GTPgammaS alone was ineffective, but GTPgammaS-loaded Cdc42 induced actin polymerization, suggesting that the response was limited by guanine nucleotide exchange. Stimulating exchange by chelating magnesium, by adding acidic phospholipids, or by adding the exchange factors Cdc24 or Dbl restored the ability of GTPgammaS to induce polymerization. The stimulation of actin polymerization did not correlate with PIP2 synthesis.
Figures
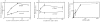
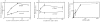
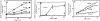


References
-
- Bokoch GMS. Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr Opin Cell Biol. 1994;6:212–218. - PubMed
-
- Bokoch GM, Bohl BP, Chuang T-H. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–31679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

