The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells
- PMID: 9234166
- PMCID: PMC2229359
- DOI: 10.1085/jgp.110.1.1
The secretion-coupled endocytosis correlates with membrane tension changes in RBL 2H3 cells
Abstract
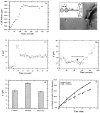
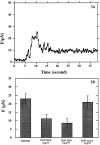
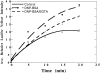
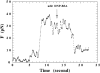
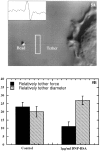
Stimulated secretion in endocrine cells and neuronal synapses causes a rise in endocytosis rates to recover the added membrane. The endocytic process involves the mechanical deformation of the membrane to produce an invagination. Studies of osmotic swelling effects on endocytosis indicate that the increased surface tension is tightly correlated to a significant decrease of endocytosis. When rat basophilic leukemia (RBL) cells are stimulated to secrete, there is a dramatic drop in the membrane tension and only small changes in membrane bending stiffness. Neither the shape change that normally accompanies secretion nor the binding of ligand without secretion causes a drop in tension. Further, tension decreases within 6 s, preceding shape change and measurable changes in endocytosis. After secretion stops, tension recovers. On the basis of these results we suggest that the physical parameter of membrane tension is a major regulator of endocytic rate in RBL cells. Low tensions would stimulate endocytosis and high tensions would stall the endocytic machinery.
Figures


References
-
- Benhamou M, Berenstein EH, Jouvin M, Siraganian RP. The receptor with high affinity for IgE on rat mast cells is a functional receptor for rat IgG2a. Mol Immunol. 1994;31:1089–1097. - PubMed
-
- Dai J, Sheetz MP. Axon membrane flows from the growth cone to the cell body. Cell. 1995b;83:693–701. - PubMed

