Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection
- PMID: 9236194
- PMCID: PMC2198992
- DOI: 10.1084/jem.186.3.421
Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection
Abstract
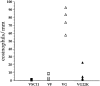
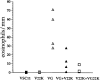
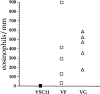
T lymphocytes play a pivotal role in the immune response during viral infections. In a murine model of experimental respiratory syncytial virus (RSV) infection, mice sensitized to either of the two major glycoproteins of RSV develop distinct patterns of cytokine secretion and lung inflammation upon subsequent RSV infection. Mice sensitized to RSV-G (attachment) glycoprotein exhibit a strong interleukin (IL)-4 and IL-5 response and develop pulmonary eosinophilia, whereas mice sensitized to RSV-F (fusion) glycoprotein develop a predominantly T helper cell (Th)1 response and pulmonary inflammation characterized by mononuclear cell infiltration. In this study, we examined the potential role of virus-specific CD8+ T cytolytic T cells on the differentiation and activation of functionally distinct CD4+ T cells specific to these viral glycoproteins. Mice primed with recombinant vaccinia virus expressing RSV-F glycoprotein mounted a strong RSV-specific, MHC class I-restricted cytolytic response, whereas priming with recombinant vaccinia virus expressing RSV-G glycoprotein failed to elicit any detectable cytolytic response. Priming for a RSV-specific CD8+ T cell response, either with a recombinant vaccinia virus expressing RSV-G glycoprotein in which a strong CD8+ T cell epitope from RSV-M2 (matrix) protein has been inserted or with a combination of vaccinia virus expressing the matrix protein and the RSV-G glycoprotein, suppressed the eosinophil recruitment into the lungs of these mice upon subsequent challenge with RSV. This reduction in pulmonary eosinophilia correlated with the suppression of Th2 type cytokine production. The importance of CD8+ T cells in this process was further supported by the results in CD8+ T cell deficient, beta 2 microglobulin KO mice. In these mice, priming to RSV-F glycoprotein (which in normal mice primed for a strong cytolytic response and a pulmonary infiltrate consisting primarily of mononuclear cells on RSV challenge) resulted in the development of marked pulmonary eosinophilia that was not seen in mice with an intact CD8+ T cell compartment. These results indicate that CD8+ T cells may play an important role in the regulation of the differentiation and activation of Th2 CD4+ T cells as well as the recruitment of eosinophils into the lungs during RSV infection.
Figures

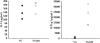


Similar articles
-
IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge.J Immunol. 2003 Feb 15;170(4):2037-45. doi: 10.4049/jimmunol.170.4.2037. J Immunol. 2003. PMID: 12574374
-
Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection.J Virol. 1997 Jan;71(1):678-85. doi: 10.1128/JVI.71.1.678-685.1997. J Virol. 1997. PMID: 8985399 Free PMC article.
-
Abundant IFN-gamma production by local T cells in respiratory syncytial virus-induced eosinophilic lung disease.J Gen Virol. 1998 Jul;79 ( Pt 7):1751-8. doi: 10.1099/0022-1317-79-7-1751. J Gen Virol. 1998. PMID: 9680139
-
Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease.Immunol Res. 2007;39(1-3):225-39. doi: 10.1007/s12026-007-0071-6. Immunol Res. 2007. PMID: 17917067 Review.
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
Cited by
-
Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response.Transgenic Res. 2000 Apr;9(2):127-35. doi: 10.1023/a:1008979525909. Transgenic Res. 2000. PMID: 10951696
-
Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice.J Virol. 2000 Feb;74(4):1614-22. doi: 10.1128/jvi.74.4.1614-1622.2000. J Virol. 2000. PMID: 10644330 Free PMC article.
-
Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3.J Virol. 2001 May;75(10):4594-603. doi: 10.1128/JVI.75.10.4594-4603.2001. J Virol. 2001. PMID: 11312329 Free PMC article.
-
Absence of vaccine-enhanced RSV disease and changes in pulmonary dendritic cells with adenovirus-based RSV vaccine.Virol J. 2011 Jul 29;8:375. doi: 10.1186/1743-422X-8-375. Virol J. 2011. PMID: 21801372 Free PMC article.
-
Simultaneous Administration of Recombinant Measles Viruses Expressing Respiratory Syncytial Virus Fusion (F) and Nucleo (N) Proteins Induced Humoral and Cellular Immune Responses in Cotton Rats.Vaccines (Basel). 2019 Mar 4;7(1):27. doi: 10.3390/vaccines7010027. Vaccines (Basel). 2019. PMID: 30836661 Free PMC article.
References
-
- Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of α/β and γ/δ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. - PubMed
-
- Braciale TJ, Braciale VL. Viral antigen presentation and MHC assembly. Semin Immunol. 1992;4:81–84. - PubMed
-
- Braciale TJ, Braciale VL. Antigen presentation: structural themes and functional variations. Immunol Today. 1991;12:124–129. - PubMed
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

