Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology
- PMID: 9236205
- PMCID: PMC2233785
- DOI: 10.1085/jgp.110.2.119
Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology
Abstract
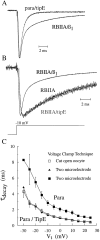
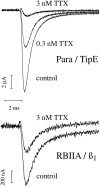
The Drosophila para sodium channel alpha subunit was expressed in Xenopus oocytes alone and in combination with tipE, a putative Drosophila sodium channel accessory subunit. Coexpression of tipE with para results in elevated levels of sodium currents and accelerated current decay. Para/TipE sodium channels have biophysical and pharmacological properties similar to those of native channels. However, the pharmacology of these channels differs from that of vertebrate sodium channels: (a) toxin II from Anemonia sulcata, which slows inactivation, binds to Para and some mammalian sodium channels with similar affinity (Kd congruent with 10 nM), but this toxin causes a 100-fold greater decrease in the rate of inactivation of Para/TipE than of mammalian channels; (b) Para sodium channels are >10-fold more sensitive to block by tetrodotoxin; and (c) modification by the pyrethroid insecticide permethrin is >100-fold more potent for Para than for rat brain type IIA sodium channels. Our results suggest that the selective toxicity of pyrethroid insecticides is due at least in part to the greater affinity of pyrethroids for insect sodium channels than for mammalian sodium channels.
Figures







Similar articles
-
Differential effects of TipE and a TipE-homologous protein on modulation of gating properties of sodium channels from Drosophila melanogaster.PLoS One. 2013 Jul 18;8(7):e67551. doi: 10.1371/journal.pone.0067551. Print 2013. PLoS One. 2013. PMID: 23874427 Free PMC article.
-
The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels.Pest Manag Sci. 2001 Oct;57(10):877-88. doi: 10.1002/ps.392. Pest Manag Sci. 2001. PMID: 11695180 Review.
-
Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Na v 1.8 sodium channels expressed in Xenopus oocytes.Toxicol Appl Pharmacol. 2006 Mar 15;211(3):233-44. doi: 10.1016/j.taap.2005.06.022. Epub 2005 Jul 26. Toxicol Appl Pharmacol. 2006. PMID: 16051293
-
Domain 2 of Drosophila para voltage-gated sodium channel confers insect properties to a rat brain channel.J Neurosci. 2002 Jun 1;22(11):4364-71. doi: 10.1523/JNEUROSCI.22-11-04364.2002. J Neurosci. 2002. PMID: 12040042 Free PMC article.
-
Neuronal ion channels as the target sites of insecticides.Pharmacol Toxicol. 1996 Jul;79(1):1-14. doi: 10.1111/j.1600-0773.1996.tb00234.x. Pharmacol Toxicol. 1996. PMID: 8841090 Review.
Cited by
-
Serotonergic modulation of visual neurons in Drosophila melanogaster.PLoS Genet. 2020 Aug 31;16(8):e1009003. doi: 10.1371/journal.pgen.1009003. eCollection 2020 Aug. PLoS Genet. 2020. PMID: 32866139 Free PMC article.
-
Ion channels: molecular targets of neuroactive insecticides.Invert Neurosci. 2005 Nov;5(3-4):119-33. doi: 10.1007/s10158-005-0004-9. Epub 2005 Oct 24. Invert Neurosci. 2005. PMID: 16172884 Review.
-
Exploring the obscure profiles of pharmacological binding sites on voltage-gated sodium channels by BmK neurotoxins.Protein Cell. 2011 Jun;2(6):437-44. doi: 10.1007/s13238-011-1064-8. Epub 2011 Jul 12. Protein Cell. 2011. PMID: 21748593 Free PMC article. Review.
-
Intron retention in mRNA encoding ancillary subunit of insect voltage-gated sodium channel modulates channel expression, gating regulation and drug sensitivity.PLoS One. 2013 Aug 15;8(8):e67290. doi: 10.1371/journal.pone.0067290. eCollection 2013. PLoS One. 2013. PMID: 23967047 Free PMC article.
-
Charge substitutions at the voltage-sensing module of domain III enhance actions of site-3 and site-4 toxins on an insect sodium channel.Insect Biochem Mol Biol. 2021 Oct;137:103625. doi: 10.1016/j.ibmb.2021.103625. Epub 2021 Aug 3. Insect Biochem Mol Biol. 2021. PMID: 34358664 Free PMC article.
References
-
- Adams ME, Olivera M. Neurotoxins: overview of an emerging research technology. Trends Neurosci. 1994;17:151–155. - PubMed
-
- Arena JP, Liu KK, Paress PS, Cully DF. Avermectin-sensitive chloride currents incuded by Caenorhabditis elegans RNA in Xenopusoocytes. Mol Pharmacol. 1991;40:368–374. - PubMed
-
- Arena JP, Liu KK, Paress PS, Schaeffer JM, Cully DF. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation with avermectin. Mol Brain Res. 1992;15:339–348. - PubMed
-
- Baden DG. Brevetoxins: unique polyether dinoflagellate toxins. FASEB J. 1989;3:1807–1817. - PubMed
-
- Bergman C, Dubois JM, Rojas E, Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim Biophys Acta. 1976;455:173–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

