Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit
- PMID: 9236207
- PMCID: PMC2233786
- DOI: 10.1085/jgp.110.2.141
Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit
Abstract
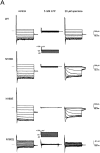
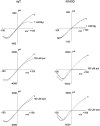
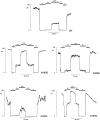
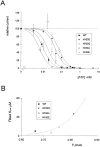
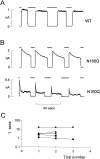
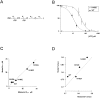
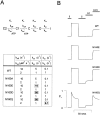
KATP channels are a functional complex of sulphonylurea receptor (SUR1, SUR2) and inward rectifier K+ (Kir6.1, Kir6.2) channel subunits. We have studied the role of the putative pore forming subunit (Kir6.2) in regulation of rectification and gating of KATP channels generated by transfection of SUR1 and Kir6.2 cDNAs in COSm6 cells. In the absence of internal polyvalent cations, the current-voltage relationship is sigmoidal. Mg2+ or spermine4+ (spm) each induces a mild inward rectification. Mutation of the asparagine at position 160 in Kir6.2 to aspartate (N160D) or glutamate (N160E) increases the degree of rectification induced by Mg2+ or spermine4+, whereas wild-type rectification is still observed after mutation to other neutral residues (alanine-N160A, glutamine-N160Q). These results are consistent with this residue lining the pore of the channel and contributing to the binding of these cations, as demonstrated for the equivalent site in homomeric ROMK1 (Kir1.1) channels. Since Kir6.2 contains no consensus ATP binding site, whereas SUR1 does, inhibition by ATP has been assumed to depend on interactions with SUR1. However, we found that the [ATP] causing half-maximal inhibition of current (Ki) was affected by mutation of N160. Channels formed from N160D or N160Q mutant subunits had lower apparent sensitivity to ATP (Ki,N160D = 46.1 microM; Ki,N160Q = 62.9 microM) than wild-type, N160E, or N160A channels (Ki = 10.4, 17.7, 6.4 microM, respectively). This might suggest that ATP binding to the channel complex was altered, although examination of channel open probabilities indicates instead that the residue at position 160 alters the ATP-independent open probability, i.e., it controls the free energy of the open state, thereby affecting the "coupling" of ATP binding to channel inhibition. The results can be interpreted in terms of a kinetic scheme whereby the residue at Kir6.2 position 160 controls the rate constants governing transitions to and from the open state, without directly affecting ATP binding or unbinding transitions.
Figures




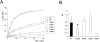
Similar articles
-
Involvement of the n-terminus of Kir6.2 in coupling to the sulphonylurea receptor.J Physiol. 1999 Jul 15;518 ( Pt 2)(Pt 2):325-36. doi: 10.1111/j.1469-7793.1999.0325p.x. J Physiol. 1999. PMID: 10381582 Free PMC article.
-
ATP inhibition of KATP channels: control of nucleotide sensitivity by the N-terminal domain of the Kir6.2 subunit.J Physiol. 1999 Feb 15;515 ( Pt 1)(Pt 1):19-30. doi: 10.1111/j.1469-7793.1999.019ad.x. J Physiol. 1999. PMID: 9925874 Free PMC article.
-
Involvement of the N-terminus of Kir6.2 in the inhibition of the KATP channel by ATP.J Physiol. 1999 Jan 1;514 ( Pt 1)(Pt 1):19-25. doi: 10.1111/j.1469-7793.1999.019af.x. J Physiol. 1999. PMID: 9831713 Free PMC article.
-
Molecular biology of adenosine triphosphate-sensitive potassium channels.Endocr Rev. 1999 Apr;20(2):101-35. doi: 10.1210/edrv.20.2.0361. Endocr Rev. 1999. PMID: 10204114 Review.
-
Diverse roles of K(ATP) channels learned from Kir6.2 genetically engineered mice.Diabetes. 2000 Mar;49(3):311-8. doi: 10.2337/diabetes.49.3.311. Diabetes. 2000. PMID: 10868950 Review.
Cited by
-
Molecular basis of inward rectification: polyamine interaction sites located by combined channel and ligand mutagenesis.J Gen Physiol. 2004 Nov;124(5):541-54. doi: 10.1085/jgp.200409159. Epub 2004 Oct 11. J Gen Physiol. 2004. PMID: 15477380 Free PMC article.
-
Stabilization of the activity of ATP-sensitive potassium channels by ion pairs formed between adjacent Kir6.2 subunits.J Gen Physiol. 2003 Aug;122(2):225-37. doi: 10.1085/jgp.200308822. J Gen Physiol. 2003. PMID: 12885877 Free PMC article.
-
The kinetic and physical basis of K(ATP) channel gating: toward a unified molecular understanding.Biophys J. 2000 May;78(5):2334-48. doi: 10.1016/S0006-3495(00)76779-8. Biophys J. 2000. PMID: 10777731 Free PMC article.
-
Congenital hyperinsulinism and glucose hypersensitivity in homozygous and heterozygous carriers of Kir6.2 (KCNJ11) mutation V290M mutation: K(ATP) channel inactivation mechanism and clinical management.Diabetes. 2011 Jan;60(1):209-17. doi: 10.2337/db10-0731. Epub 2010 Oct 27. Diabetes. 2011. PMID: 20980454 Free PMC article.
-
Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore.J Gen Physiol. 2003 Dec;122(6):795-804. doi: 10.1085/jgp.200308953. J Gen Physiol. 2003. PMID: 14638936 Free PMC article.
References
-
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. - PubMed
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the β-cell high affinity sulfonylurea receptor: a regulator of insulin secretion. Science (Wash DC) 1995;268:423–426. - PubMed
-
- Bianchi L, Roy ML, Taglialatela M, Lundgren DW, Brown AM, Ficker E. Regulation by spermine of native inward rectifier K+channels in RBL-1 cells. J Biol Chem. 1996;271:6114–6121. - PubMed
-
- Cameron JS, Kimura S, Jackson-Burns DA, Smith DB, Bassett AL. ATP-sensitive K+channels are altered in hypertrophied ventricular myocytes. Am J Physiol. 1988;255:H1254–H1258. - PubMed
-
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. - PubMed

