Cyclic GMP-gated channels in a sympathetic neuron cell line
- PMID: 9236208
- PMCID: PMC2233783
- DOI: 10.1085/jgp.110.2.155
Cyclic GMP-gated channels in a sympathetic neuron cell line
Abstract
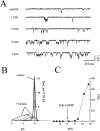
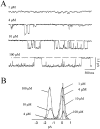
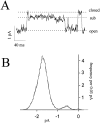
The stimulation of IP3 production by muscarinic agonists causes both intracellular Ca2+ release and activation of a voltage-independent cation current in differentiated N1E-115 cells, a neuroblastoma cell line derived from mouse sympathetic ganglia. Earlier work showed that the membrane current requires an increase in 3',5'-cyclic guanosine monophosphate (cGMP) produced through the NO-synthase/guanylyl cyclase cascade and suggested that the cells may express cyclic nucleotide-gated ion channels. This was tested using patch clamp methods. The membrane permeable cGMP analogue, 8-br-cGMP, activates Na+ permeable channels in cell attached patches. Single channel currents were recorded in excised patches bathed in symmetrical Na+ solutions. cGMP-dependent single channel activity consists of prolonged bursts of rapid openings and closings that continue without desensitization. The rate of occurrence of bursts as well as the burst length increase with cGMP concentration. The unitary conductance in symmetrical 160 mM Na+ is 47 pS and is independent of voltage in the range -50 to +50 mV. There is no apparent effect of voltage on opening probability. The dose response curve relating cGMP concentration to channel opening probability is fit by the Hill equation assuming an apparent KD of 10 microm and a Hill coefficient of 2. In contrast, cAMP failed to activate the channel at concentrations as high as 100 microm. Cyclic nucleotide gated (CNG) channels in N1E-115 cells share a number of properties with CNG channels in sensory receptors. Their presence in neuronal cells provides a mechanism by which activation of the NO/cGMP pathway by G-protein-coupled neurotransmitter receptors can directly modify Ca2+ influx and electrical excitability. In N1E-115 cells, Ca2+ entry by this pathway is necessary to refill the IP3-sensitive intracellular Ca2+ pool during repeated stimulation and CNG channels may play a similar role in other neurons.
Figures





Similar articles
-
Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein.Circ Res. 1998 Mar 23;82(5):557-65. doi: 10.1161/01.res.82.5.557. Circ Res. 1998. PMID: 9529160
-
cGMP/protein kinase G-dependent inhibition of N-type Ca2+ channels induced by nitric oxide in human neuroblastoma IMR32 cells.J Neurosci. 2002 Sep 1;22(17):7485-92. doi: 10.1523/JNEUROSCI.22-17-07485.2002. J Neurosci. 2002. PMID: 12196571 Free PMC article.
-
Gating by cyclic GMP and voltage in the alpha subunit of the cyclic GMP-gated channel from rod photoreceptors.J Gen Physiol. 1999 Oct;114(4):477-90. doi: 10.1085/jgp.114.4.477. J Gen Physiol. 1999. PMID: 10498668 Free PMC article.
-
Cyclic nucleotide-gated channels--mediators of NO:cGMP-regulated processes.Naunyn Schmiedebergs Arch Pharmacol. 1998 Jul;358(1):140-4. doi: 10.1007/pl00005235. Naunyn Schmiedebergs Arch Pharmacol. 1998. PMID: 9721016 Review.
-
Molecular and pharmacological analysis of cyclic nucleotide-gated channel function in the central nervous system.Prog Neurobiol. 1998 Oct;56(1):37-64. doi: 10.1016/s0301-0082(98)00029-x. Prog Neurobiol. 1998. PMID: 9723130 Review.
Cited by
-
Semaphorin 3A inhibits growth of adult sympathetic and parasympathetic neurones via distinct cyclic nucleotide signalling pathways.Br J Pharmacol. 2011 Mar;162(5):1083-95. doi: 10.1111/j.1476-5381.2010.01108.x. Br J Pharmacol. 2011. PMID: 21054346 Free PMC article.
-
Long-term depression-related tau phosphorylation is enhanced by methylene blue in healthy rat hippocampus.Pharmacol Rep. 2021 Jun;73(3):828-840. doi: 10.1007/s43440-021-00254-y. Epub 2021 Apr 2. Pharmacol Rep. 2021. PMID: 33797746
-
New molecular players in capacitative Ca2+ entry.J Cell Sci. 2007 Jun 15;120(Pt 12):1959-65. doi: 10.1242/jcs.03462. Epub 2007 May 3. J Cell Sci. 2007. PMID: 17478524 Free PMC article.
-
Single-molecule imaging with cell-derived nanovesicles reveals early binding dynamics at a cyclic nucleotide-gated ion channel.Nat Commun. 2021 Nov 9;12(1):6459. doi: 10.1038/s41467-021-26816-5. Nat Commun. 2021. PMID: 34753946 Free PMC article.
-
Ca2+ permeation in cyclic nucleotide-gated channels.EMBO J. 1999 Jan 4;18(1):131-44. doi: 10.1093/emboj/18.1.131. EMBO J. 1999. PMID: 9878057 Free PMC article.
References
-
- Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GS, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–165. - PubMed
-
- Arroyo CM, Forray CM. Activation of cyclic GMP formation in mouse neuroblastoma cells by a labile nitroxyl radical: an electron paramagnetic resonance spin trapping study. Euro J Pharm Mol Pharm. 1991;6:157–161. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature (Lond) 1993;361:315–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

