Inward rectification in ClC-0 chloride channels caused by mutations in several protein regions
- PMID: 9236209
- PMCID: PMC2233784
- DOI: 10.1085/jgp.110.2.165
Inward rectification in ClC-0 chloride channels caused by mutations in several protein regions
Abstract
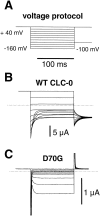
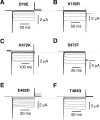
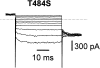
Several cloned ClC-type Cl- channels open and close in a voltage-dependent manner. The Torpedo electric organ Cl- channel, ClC-0, is the best studied member of this gene family. ClC-0 is gated by a fast and a slow gating mechanism of opposite voltage direction. Fast gating is dependent on voltage and on the external and internal Cl- concentration, and it has been proposed that the permeant anion serves as the gating charge in ClC-0 (Pusch, M., U. Ludewig, A. Rehfeldt, and T.J. Jentsch. 1995. Nature (Lond.). 373:527-531). The deactivation at negative voltages of the muscular ClC-1 channel is similar but not identical to ClC-0. Different from the extrinsic voltage dependence suggested for ClC-0, an intrinsic voltage sensor had been proposed to underlie the voltage dependence in ClC-1 (Fahlke, C., R. Rüdel, N. Mitrovic, M. Zhou, and A.L. George. 1995. Neuron. 15:463-472; Fahlke, C., A. Rosenbohm, N. Mitrovic, A.L. George, and R. Rüdel. 1996. Biophys. J. 71:695-706). The gating model for ClC-1 was partially based on the properties of a point-mutation found in recessice myotonia (D136G). Here we investigate the functional effects of mutating the corresponding residue in ClC-0 (D70). Both the corresponding charge neutralization (D70G) and a charge conserving mutation (D70E) led to an inwardly rectifying phenotype resembling that of ClC-1 (D136G). Several other mutations at very different positions in ClC-0 (K165R, H472K, S475T, E482D, T484S, T484Q), however, also led to a similar phenotype. In one of these mutants (T484S) the typical wild-type gating, characterized by a deactivation at negative voltages, can be partially restored by using external perchlorate (ClO4-) solutions. We conclude that gating in ClC-0 and ClC-1 is due to similar mechanisms. The negative charge at position 70 in ClC-0 does not specifically confer the voltage sensitivity in ClC-channels, and there is no need to postulate an intrinsic voltage sensor in ClC-channels.
Figures

Similar articles
-
Chloride dependence of hyperpolarization-activated chloride channel gates.J Physiol. 1999 Mar 1;515 ( Pt 2)(Pt 2):341-53. doi: 10.1111/j.1469-7793.1999.341ac.x. J Physiol. 1999. PMID: 10050002 Free PMC article.
-
Analysis of a protein region involved in permeation and gating of the voltage-gated Torpedo chloride channel ClC-0.J Physiol. 1997 Feb 1;498 ( Pt 3)(Pt 3):691-702. doi: 10.1113/jphysiol.1997.sp021893. J Physiol. 1997. PMID: 9051580 Free PMC article.
-
Fast and slow gating relaxations in the muscle chloride channel CLC-1.J Gen Physiol. 2000 Sep;116(3):433-44. doi: 10.1085/jgp.116.3.433. J Gen Physiol. 2000. PMID: 10962018 Free PMC article.
-
Properties of voltage-gated chloride channels of the ClC gene family.J Physiol. 1995 Jan;482(P):19S-25S. doi: 10.1113/jphysiol.1995.sp020560. J Physiol. 1995. PMID: 7730971 Free PMC article. Review.
-
Coupling gating with ion permeation in ClC channels.Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review.
Cited by
-
Chloride dependence of hyperpolarization-activated chloride channel gates.J Physiol. 1999 Mar 1;515 ( Pt 2)(Pt 2):341-53. doi: 10.1111/j.1469-7793.1999.341ac.x. J Physiol. 1999. PMID: 10050002 Free PMC article.
-
A tale of two CLCs: biophysical insights toward understanding ClC-5 and ClC-7 function in endosomes and lysosomes.J Physiol. 2015 Sep 15;593(18):4139-50. doi: 10.1113/JP270604. Epub 2015 Jun 26. J Physiol. 2015. PMID: 26036722 Free PMC article. Review.
-
Cysteine accessibility in ClC-0 supports conservation of the ClC intracellular vestibule.J Gen Physiol. 2005 Jun;125(6):601-17. doi: 10.1085/jgp.200509258. Epub 2005 May 16. J Gen Physiol. 2005. PMID: 15897295 Free PMC article.
-
Substrate-driven conformational changes in ClC-ec1 observed by fluorine NMR.EMBO J. 2009 Oct 21;28(20):3090-102. doi: 10.1038/emboj.2009.259. Epub 2009 Sep 10. EMBO J. 2009. PMID: 19745816 Free PMC article.
-
Multiple discrete transitions underlie voltage-dependent activation in CLC Cl(-)/H(+) antiporters.Biophys J. 2014 Sep 16;107(6):L13-5. doi: 10.1016/j.bpj.2014.07.063. Biophys J. 2014. PMID: 25229156 Free PMC article.
References
-
- Brandt S, Jentsch TJ. ClC-6 and ClC-7 are two novel broadly expressed members of the ClC chloride channel family. FEBS Lett. 1995;377:15–20. - PubMed
-
- Fahlke C, Rüdel R, Mitrovic N, Zhou M, George AL. An aspartic residue important for voltage-dependent gating of human muscle chloride channels. Neuron. 1995;15:463–472. - PubMed
-
- Greene JR, Brown NH, DiDomenico BJ, Kaplan J, Eide DJ. The GEF-1 gene of Saccharomyces cerevisiaeencodes an integral membrane protein; mutations in which have effects on respiration and iron-limited growth. Mol Gen Genet. 1993;241:542–553. - PubMed

