NO3--induced pH changes in mammalian cells. Evidence for an NO3--H+ cotransporter
- PMID: 9236211
- PMCID: PMC2233787
- DOI: 10.1085/jgp.110.2.185
NO3--induced pH changes in mammalian cells. Evidence for an NO3--H+ cotransporter
Abstract
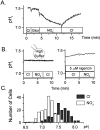
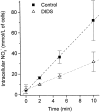
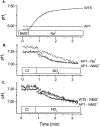
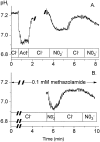
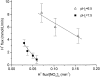
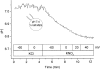
The effect of NO3- on intracellular pH (pHi) was assessed microfluorimetrically in mammalian cells in culture. In cells of human, hamster, and murine origin addition of extracellular NO3- induced an intracellular acidification. This acidification was eliminated when the cytosolic pH was clamped using ionophores or by perfusing the cytosol with highly buffered solutions using patch-pipettes, ruling out spectroscopic artifacts. The NO3-- induced pH change was not due to modulation of Na+/H+ exchange, since it was also observed in Na+/H+ antiport-deficient mutants. Though NO3- is known to inhibit vacuolar-type (V) H+-ATPases, this effect was not responsible for the acidification since it persisted in the presence of the potent V-ATPase inhibitor bafilomycin A1. NO3-/HCO3- exchange as the underlying mechanism was ruled out because acidification occurred despite nominal removal of HCO3-, despite inhibition of the anion exchanger with disulfonic stilbenes and in HEK 293 cells, which seemingly lack anion exchangers (Lee, B. S., R.B. Gunn, and R.R. Kopito. 1991. J. Biol. Chem. 266:11448- 11454). Accumulation of intracellular NO3-, measured by the Greiss method after reduction to NO2-, indicated that the anion is translocated into the cells along with the movement of acid equivalents. The simplest model to explain these observations is the cotransport of NO3- with H+ (or the equivalent counter-transport of NO3- for OH-). The transporter appears to be bi-directional, operating in the forward as well as reverse directions. A rough estimate of the fluxes of NO3- and acid equivalents suggests a one-to-one stoichiometry. Accordingly, the rate of transport was unaffected by sizable changes in transmembrane potential. The cytosolic acidification was a saturable function of the extracellular concentration of NO3- and was accentuated by acidification of the extracellular space. The putative NO3--H+ cotransport was inhibited markedly by ethacrynic acid and by alpha-cyano-4-hydroxycinnamate, but only marginally by 4, 4'-diisothiocyanostilbene-2,2' disulfonate or by p-chloromercuribenzene sulfonate. The transporter responsible for NO3--induced pH changes in mammalian cells may be related, though not identical, to the NO3--H+ cotransporter described in Arabidopsis and Aspergillus. The mammalian cotransporter may be important in eliminating the products of NO metabolism, particularly in cells that generate vast amounts of this messenger. By cotransporting NO3- with H+ the cells would additionally eliminate acid equivalents from activated cells that are metabolizing actively, without added energetic investment and with minimal disruption of the transmembrane potential, inasmuch as the cotransporter is likely electroneutral.
Figures



Similar articles
-
The mammalian Na+/H+ antiporters NHE-1, NHE-2, and NHE-3 are electroneutral and voltage independent, but can couple to an H+ conductance.J Gen Physiol. 1995 Jul;106(1):85-111. doi: 10.1085/jgp.106.1.85. J Gen Physiol. 1995. PMID: 7494140 Free PMC article.
-
Cytosolic pH regulation in osteoblasts. Regulation of anion exchange by intracellular pH and Ca2+ ions.J Gen Physiol. 1990 Jan;95(1):121-45. doi: 10.1085/jgp.95.1.121. J Gen Physiol. 1990. PMID: 2299328 Free PMC article.
-
Anion inhibition of the proton pump in rat liver multivesicular bodies.J Biol Chem. 1986 Dec 5;261(34):15941-8. J Biol Chem. 1986. PMID: 3023335
-
Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation.Biochim Biophys Acta. 1989 Jan 18;988(1):73-97. doi: 10.1016/0304-4157(89)90004-x. Biochim Biophys Acta. 1989. PMID: 2535787 Review. No abstract available.
-
Intracellular pH Control by Membrane Transport in Mammalian Cells. Insights Into the Selective Advantages of Functional Redundancy.Front Mol Biosci. 2022 Feb 18;9:825028. doi: 10.3389/fmolb.2022.825028. eCollection 2022. Front Mol Biosci. 2022. PMID: 35252350 Free PMC article. Review.
Cited by
-
S-Nitroso-N-acetyl-L-cysteine ethyl ester (SNACET) and N-acetyl-L-cysteine ethyl ester (NACET)-Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO, H2S and GSH suppliers and as antioxidants: Results and overview.J Pharm Anal. 2018 Feb;8(1):1-9. doi: 10.1016/j.jpha.2017.12.003. Epub 2017 Dec 13. J Pharm Anal. 2018. PMID: 29568662 Free PMC article. Review.
-
NitrOFF: An engineered fluorescent biosensor to illuminate nitrate transport in living cells.bioRxiv [Preprint]. 2025 Mar 23:2025.03.22.644677. doi: 10.1101/2025.03.22.644677. bioRxiv. 2025. PMID: 40166251 Free PMC article. Preprint.
-
Chronic inorganic nitrate supplementation does not improve metabolic health and worsens disease progression in mice with diet-induced obesity.Am J Physiol Endocrinol Metab. 2025 Jan 1;328(1):E69-E91. doi: 10.1152/ajpendo.00256.2024. Epub 2024 Dec 9. Am J Physiol Endocrinol Metab. 2025. PMID: 39653040 Free PMC article.
-
Pulsed 3.5 GHz high power microwaves irradiation on physiological solution and their biological evaluation on human cell lines.Sci Rep. 2021 Apr 19;11(1):8475. doi: 10.1038/s41598-021-88078-x. Sci Rep. 2021. PMID: 33875781 Free PMC article.
-
Missense mutation T485S alters NBCe1-A electrogenicity causing proximal renal tubular acidosis.Am J Physiol Cell Physiol. 2013 Aug 15;305(4):C392-405. doi: 10.1152/ajpcell.00044.2013. Epub 2013 May 1. Am J Physiol Cell Physiol. 2013. PMID: 23636456 Free PMC article.
References
-
- Anggard E. Nitric oxide: mediator, murderer and medicine. Lancet. 1994;343:1199–1206. - PubMed
-
- Begault B, Edelman A. A nucleotide-regulated Cl−/ OH−anion exchanger in endoplasmic reticulum-enriched pig pancreatic microsomes. Biochim Biophys Acta. 1993;1146:183–190. - PubMed
-
- Bruce LJ, Anstee DJ, Spring FA, Tanner MJA. Band 3 Memphis variant II. J Biol Chem. 1994a;23:16155–16158. - PubMed
-
- Bruce LJ, Groves JD, Okubo Y, Thilaganathan B, Tanner MJA. Altered band 3 structure and function in glycopho-rin A- and B-deficient (MkMk) red blood cells. Blood. 1994b;84:916–922. - PubMed
-
- Cabantchik ZI, Knauf PA, Rothstein A. The anion transport system of the red blood cell. Biochim Biophys Acta. 1978;515:239–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

