Apolipoprotein E binds to and potentiates the biological activity of ciliary neurotrophic factor
- PMID: 9236223
- PMCID: PMC6568355
- DOI: 10.1523/JNEUROSCI.17-16-06114.1997
Apolipoprotein E binds to and potentiates the biological activity of ciliary neurotrophic factor
Abstract
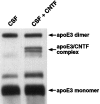
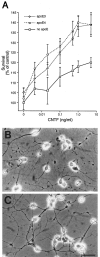
Expression of apolipoprotein E (apoE) and ciliary neurotrophic factor (CNTF), a pleiotropic neuron survival factor, increases in the CNS in response to injury. Although CNTF is believed to act as a survival factor after injury in the CNS, the functions of apoE in the CNS remain mainly unknown. Similarities between apoE and CNTF, including coinciding patterns of postinjury expression, extracellular localization, homologous tertiary structure, and ability to form homodimers led us to examine the possibility that apoE and CNTF directly associate and thereby facilitate the neurotrophic activity of CNTF. We identified two binding interactions between apoE and CNTF: (1) reversible binding of both the apoE3 and apoE4 isoforms to CNTF under nondenaturing conditions, and (2) a higher avidity, SDS-stable binding of apoE3 with CNTF. Purified lipid-free apoE, as well as apoE in cerebrospinal fluid, binds CNTF. We demonstrate here that the survival-promoting activity of CNTF on cultured hippocampal neurons is potentiated by apoE. In the absence of apoE, survival of hippocampal neurons with 1 ng/ml CNTF was 20% above control survival values. In contrast, in the presence of apoE, survival of hippocampal neurons with 1 ng/ml CNTF was 40% above control survival values. These data, which indicate a novel function for apoE in the nervous system, support the hypothesis that apoE secreted locally at sites of injury can facilitate neural repair by promoting the activity of certain growth factors, in particular CNTF.
Figures



Similar articles
-
Recombinant human ciliary neurotrophic factor alters the threshold of hippocampal pyramidal neuron sensitivity to excitotoxin damage: synergistic effects of monosialogangliosides.J Neurosci Res. 1992 Oct;33(2):330-7. doi: 10.1002/jnr.490330217. J Neurosci Res. 1992. PMID: 1360545
-
Ciliary neurotrophic factor enhances neuronal survival in embryonic rat hippocampal cultures.J Neurosci. 1991 Oct;11(10):3124-34. doi: 10.1523/JNEUROSCI.11-10-03124.1991. J Neurosci. 1991. PMID: 1941077 Free PMC article.
-
Leukemia inhibitory factor and neurotrophins support overlapping populations of rat nodose sensory neurons in culture.Dev Biol. 1994 Feb;161(2):338-44. doi: 10.1006/dbio.1994.1035. Dev Biol. 1994. PMID: 8313987
-
Ciliary neurotrophic factor: a review.Pharmacol Ther. 1994 Aug;63(2):187-98. doi: 10.1016/0163-7258(94)90045-0. Pharmacol Ther. 1994. PMID: 7809179 Review.
-
Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival.J Cell Sci Suppl. 1991;15:103-9. doi: 10.1242/jcs.1991.supplement_15.14. J Cell Sci Suppl. 1991. PMID: 1824101 Review.
Cited by
-
Isoform-specific effect of apolipoprotein E on cell survival and beta-amyloid-induced toxicity in rat hippocampal pyramidal neuronal cultures.J Neurosci. 1998 Jan 1;18(1):195-204. doi: 10.1523/JNEUROSCI.18-01-00195.1998. J Neurosci. 1998. PMID: 9412500 Free PMC article.
-
Apolipoprotein E imbalance in the cerebrospinal fluid of Alzheimer's disease patients.Alzheimers Res Ther. 2022 Nov 2;14(1):161. doi: 10.1186/s13195-022-01108-2. Alzheimers Res Ther. 2022. PMID: 36324176 Free PMC article.
-
Apolipoprotein E polymorphism in patients with neuropsychiatric SLE.Clin Rheumatol. 2004 Apr;23(2):97-101. doi: 10.1007/s10067-003-0796-0. Epub 2004 Jan 29. Clin Rheumatol. 2004. PMID: 15045621
-
Apolipoprotein E knockout mice have accentuated malnutrition with mucosal disruption and blunted insulin-like growth factor I responses to refeeding.Nutr Res. 2006 Aug;26(8):427-435. doi: 10.1016/j.nutres.2006.06.020. Nutr Res. 2006. PMID: 25210213 Free PMC article.
-
Molecular signatures in post-mortem brain tissue of younger individuals at high risk for Alzheimer's disease as based on APOE genotype.Mol Psychiatry. 2011 Aug;16(8):836-47. doi: 10.1038/mp.2010.57. Epub 2010 May 18. Mol Psychiatry. 2011. PMID: 20479757 Free PMC article.
References
-
- Adler R. Ciliary neurotrophic factor as an injury factor. Curr Opin Neurobiol. 1993;3:785–789. - PubMed
-
- Alberts AJ, Graffagnino C, McClenny C, Delong WJ, Strittmatter WJ, Saunders AM, Roses AD. Effect of apoE genotype on survival after intracerebral hemorrhage. Stroke. 1996;27:183A. - PubMed
-
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. - PubMed
-
- Banker GA, Cowen WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
