N-Type calcium channels in the developing rat hippocampus: subunit, complex, and regional expression
- PMID: 9236227
- PMCID: PMC6568343
- DOI: 10.1523/JNEUROSCI.17-16-06152.1997
N-Type calcium channels in the developing rat hippocampus: subunit, complex, and regional expression
Abstract
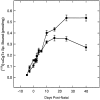
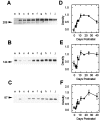
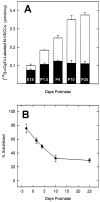
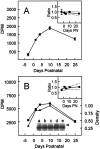
The expression of multiple classes of voltage-dependent calcium channels (VDCCs) allows neurons to tailor calcium signaling to functionally discrete cellular regions. In the developing hippocampus a central issue is whether the expression of VDCC subtypes plays a role in key phases such as migration and synaptogenesis. Using radioligand binding and immunoblotting, we show that some N-type VDCCs exist before birth, consistent with a role in migration; however, most N-VDCC subunit expression is postnatal, coinciding with synaptogenesis. Immunoprecipitation studies indicate that the increased expression of N-VDCCs in early development occurs without subunit switching because there is no change in the fraction of beta3 subunits in the N-VDCC alpha1B-beta3 heteromers. Fluorescence imaging of cell surface N-VDCCs during this period reveals that N-VDCCs are expressed on somata before dendrites and that this expression is asynchronous between different subfields of the hippocampus (CA3-CA4 before CA1-CA2 and dentate gyrus). Our data argue that N-VDCC expression is an important cue in the genesis of synaptic transmission in discrete hippocampal subfields.
Figures



Similar articles
-
N-type Ca2+ channels are located on somata, dendrites, and a subpopulation of dendritic spines on live hippocampal pyramidal neurons.J Neurosci. 1994 Nov;14(11 Pt 2):6815-24. doi: 10.1523/JNEUROSCI.14-11-06815.1994. J Neurosci. 1994. PMID: 7525892 Free PMC article.
-
The use of invertebrate peptide toxins to establish Ca2+ channel identity of CA3-CA1 neurotransmission in rat hippocampal slices.Eur J Pharmacol. 1996 Jun 13;306(1-3):41-50. doi: 10.1016/0014-2999(96)00195-1. Eur J Pharmacol. 1996. PMID: 8813613
-
Age-related changes in the subtypes of voltage-dependent calcium channels in rat brain cortical synapses.Neurosci Res. 2001 Feb;39(2):213-20. doi: 10.1016/s0168-0102(00)00212-1. Neurosci Res. 2001. PMID: 11223467
-
Differential expression and association of calcium channel subunits in development and disease.J Bioenerg Biomembr. 1998 Aug;30(4):409-18. doi: 10.1023/a:1021997924473. J Bioenerg Biomembr. 1998. PMID: 9758336 Review.
-
Neuronal populations of rat cerebral cortex and hippocampus expressed a higher density of L-type Ca 2+ channel than corresponding cerebral vessels.Clin Exp Hypertens. 2002 Oct-Nov;24(7-8):715-26. doi: 10.1081/ceh-120015347. Clin Exp Hypertens. 2002. PMID: 12450246 Review.
Cited by
-
Vulnerability of central neurons to secondary insults after in vitro mechanical stretch.J Neurosci. 2004 Sep 15;24(37):8106-23. doi: 10.1523/JNEUROSCI.1362-04.2004. J Neurosci. 2004. PMID: 15371512 Free PMC article.
-
Neurotoxin-Derived Optical Probes for Biological and Medical Imaging.Mol Imaging Biol. 2023 Oct;25(5):799-814. doi: 10.1007/s11307-023-01838-1. Epub 2023 Jul 19. Mol Imaging Biol. 2023. PMID: 37468801 Free PMC article. Review.
-
Shining a Light on Venom-Peptide Receptors: Venom Peptides as Targeted Agents for In Vivo Molecular Imaging.Toxins (Basel). 2024 Jul 4;16(7):307. doi: 10.3390/toxins16070307. Toxins (Basel). 2024. PMID: 39057947 Free PMC article. Review.
-
Ion channels and ionotropic receptors in human embryonic stem cell derived neural progenitors.Neuroscience. 2011 Sep 29;192:793-805. doi: 10.1016/j.neuroscience.2011.04.039. Epub 2011 Jun 7. Neuroscience. 2011. PMID: 21672611 Free PMC article.
-
Modelling zinc changes at the hippocampal mossy fiber synaptic cleft.J Comput Neurosci. 2016 Dec;41(3):323-337. doi: 10.1007/s10827-016-0620-x. Epub 2016 Oct 1. J Comput Neurosci. 2016. PMID: 27696002
References
-
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990a;301:325–342. - PubMed
-
- Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J Comp Neurol. 1990b;301:343–364. - PubMed
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990c;301:365–381. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
