Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil
- PMID: 9236229
- PMCID: PMC6568367
- DOI: 10.1523/JNEUROSCI.17-16-06179.1997
Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil
Abstract
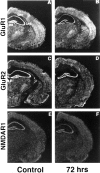
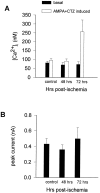
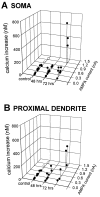
Transient, severe forebrain or global ischemia leads to delayed cell death of pyramidal neurons in the hippocampal CA1. The precise molecular mechanisms underlying neuronal cell death after global ischemia are as yet unknown. Glutamate receptor-mediated Ca2+ influx is thought to play a critical role in this cell death. In situ hybridization revealed that the expression of mRNA encoding GluR2 (the subunit that limits Ca2+ permeability of AMPA-type glutamate receptors) was markedly and specifically reduced in gerbil CA1 pyramidal neurons after global ischemia but before the onset of neurodegeneration. To determine whether the change in GluR2 expression is functionally significant, we examined the AMPA receptor-mediated rise in cytoplasmic free Ca2+ level ([Ca2+]i) in individual CA1 pyramidal neurons by optical imaging with the Ca2+ indicator dye fura-2 and by intracellular recording. Seventy-two hours after ischemia, CA1 neurons that retained the ability to fire action potentials exhibited a greatly enhanced AMPA-elicited rise in [Ca2+]i. Basal [Ca2+]i in these neurons was unchanged. These findings provide evidence for Ca2+ entry directly through AMPA receptors in pyramidal neurons destined to die. Downregulation of GluR2 gene expression and an increase in Ca2+ influx through AMPA receptors in response to endogenous glutamate are likely to contribute to the delayed neuronal death after global ischemia.
Figures

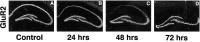



Similar articles
-
Aurintricarboxylic acid prevents GLUR2 mRNA down-regulation and delayed neurodegeneration in hippocampal CA1 neurons of gerbil after global ischemia.Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):7115-20. doi: 10.1073/pnas.95.12.7115. Proc Natl Acad Sci U S A. 1998. PMID: 9618548 Free PMC article.
-
Knockdown of AMPA receptor GluR2 expression causes delayed neurodegeneration and increases damage by sublethal ischemia in hippocampal CA1 and CA3 neurons.J Neurosci. 1999 Nov 1;19(21):9218-27. doi: 10.1523/JNEUROSCI.19-21-09218.1999. J Neurosci. 1999. PMID: 10531425 Free PMC article.
-
Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia.Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2906-10. doi: 10.1073/pnas.2628027100. Epub 2003 Feb 26. Proc Natl Acad Sci U S A. 2003. PMID: 12606709 Free PMC article.
-
The AMPAR subunit GluR2: still front and center-stage.Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
-
AMPA receptor subunit GluR2 gates injurious signals in ischemic stroke.Mol Neurobiol. 2005 Oct;32(2):145-55. doi: 10.1385/MN:32:2:145. Mol Neurobiol. 2005. PMID: 16215279 Review.
Cited by
-
Ischemic insults derepress the gene silencer REST in neurons destined to die.J Neurosci. 2003 Mar 15;23(6):2112-21. doi: 10.1523/JNEUROSCI.23-06-02112.2003. J Neurosci. 2003. PMID: 12657670 Free PMC article.
-
The sodium channel blocker RS100642 reverses down-regulation of the sodium channel alpha-subunit Na(v) 1.1 expression caused by transient ischemic brain injury in rats.Neurotox Res. 2003;5(4):245-53. doi: 10.1007/BF03033382. Neurotox Res. 2003. PMID: 12835116
-
LiCl Pretreatment Ameliorates Adolescent Methamphetamine Exposure-Induced Long-Term Alterations in Behavior and Hippocampal Ultrastructure in Adulthood in Mice.Int J Neuropsychopharmacol. 2019 Apr 1;22(4):303-316. doi: 10.1093/ijnp/pyz001. Int J Neuropsychopharmacol. 2019. PMID: 30649326 Free PMC article.
-
Delayed neuronal cell death in brainstem after transient brainstem ischemia in gerbils.BMC Neurosci. 2010 Sep 14;11:115. doi: 10.1186/1471-2202-11-115. BMC Neurosci. 2010. PMID: 20840766 Free PMC article.
-
PICK1-mediated glutamate receptor subunit 2 (GluR2) trafficking contributes to cell death in oxygen/glucose-deprived hippocampal neurons.J Biol Chem. 2009 May 22;284(21):14230-5. doi: 10.1074/jbc.M901203200. Epub 2009 Mar 25. J Biol Chem. 2009. PMID: 19321442 Free PMC article.
References
-
- Aoki M, Abe K, Kawagoe J, Nakamura S, Kogure K. Acceleration of HSP70 and HSC70 heat shock gene expression following transient ischemia in the preconditioned gerbil hippocampus. J Cereb Blood Flow Metab. 1993;13:781–888. - PubMed
-
- Baude A, Nusser Z, McIlHinney RAJ, Molnar E, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
-
- Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. - PubMed
-
- Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
