Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion
- PMID: 9236232
- PMCID: PMC2693053
- DOI: 10.1523/JNEUROSCI.17-16-06213.1997
Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion
Abstract
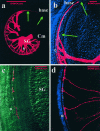
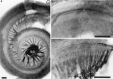
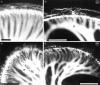
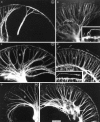
Essential functions of neurotrophin 3 (NT-3) in regulating afferent and efferent innervation of the cochlea have been characterized by comparison of normal and NT-3 mutant mice. NT-3 deficiency has striking, region-specific effects, with complete loss of sensory neurons in the basal turn and dramatic but incomplete neuronal loss in the middle and apical turns. The sensory innervation of inner and outer hair cells was reorganized in mutant animals. Instead of a strictly radial pattern of innervation, the axons of remaining sensory neurons projected spirally along the row of inner hair cells to innervate even the most basal inner hair cells. Innervation of outer hair cells was strongly reduced overall and was not detected in the basal turn. The presence of fibers extending to both inner and outer hair cells suggests that subsets of types I and II sensory neurons survive in the absence of NT-3. Likewise, projections of the cochlea to auditory nuclei of the brainstem were attenuated but otherwise present. Equally striking changes in efferent innervation were observed in mutant animals that closely mimicked the abnormal sensory innervation pattern. Despite these impressive innervation deficiencies, the morphology of the organ of Corti and the development of inner and outer hair cells appeared comparatively normal.
Figures






References
-
- Berglund AM, Benson TE, Brown MC. Synapses from labeled type II axons in the mouse cochlear nucleus. Hear Res. 1996;94:31–46. - PubMed
-
- Bianchi LM, Cohan CS. Effects of the neurotrophins and CNTF on developing statoacoustic neurons: comparison with an otocyst-derived factor. Dev Biol. 1993;159:353–365. - PubMed
-
- Bianchi LM, Conover JC, Fritzsch B, De Chiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
