Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex
- PMID: 9236251
- PMCID: PMC6568348
- DOI: 10.1523/JNEUROSCI.17-16-06434.1997
Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex
Abstract
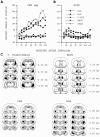
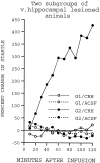
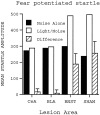
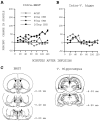
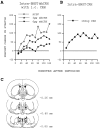
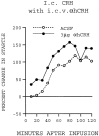
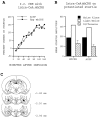
Previously, we demonstrated that transection of the fimbria/fornix blocked the excitatory effect of corticotropin-releasing hormone (CRH) on startle (CRH-enhanced startle), suggesting that the hippocampus and its efferent target areas that communicate via the fimbria may be critically involved in CRH-enhanced startle. The bed nucleus of the stria terminalis (BNST) receives direct projections from the ventral hippocampus via the fimbria/fornix. Therefore, the role of the ventral hippocampus, the BNST, and the amygdala in CRH-enhanced startle was investigated. NMDA lesions of the BNST completely blocked CRH-enhanced startle, whereas chemical lesions of the ventral hippocampus and the amygdala failed to block CRH-enhanced startle. However, the same amygdala-lesioned animals showed a complete blockade of fear-potentiated startle, a conditioned fear response sensitive to manipulations of the amygdala. In contrast, BNST-lesioned rats had normal fear-potentiated startle. This indicates a double dissociation between the BNST and the amygdala in two different paradigms that enhance startle amplitude. Microinfusions of CRH into the BNST, but not into the ventral hippocampus, mimicked intracerebroventricular CRH effects. Furthermore, infusion of a CRH antagonist into the BNST blocked CRH-enhanced startle in a dose-dependent manner. Control studies showed that this blockade did not result from either leakage of the antagonist into the ventricular system or a local anesthetic effect caused by infusion of the antagonist into the BNST. The present studies strongly suggest that CRH in the CSF can activate the BNST, which could lead to activation of brainstem and hypothalamic BNST target areas involved in anxiety and stress responses.
Figures
References
-
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 495–578.
-
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 443–493.
-
- Arató M, Bánki C, Bissette G, Nemeroff CB. Elevated CSF CRH in suicide victims. Biol Psychiatry. 1989;25:255–359. - PubMed
-
- Arnold FJL, Bueno MD, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–390. - PubMed
-
- Bánki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord. 1992a;25:39–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
