Measurements of attractive forces between proteins and end-grafted poly(ethylene glycol) chains
- PMID: 9237988
- PMCID: PMC22932
- DOI: 10.1073/pnas.94.16.8399
Measurements of attractive forces between proteins and end-grafted poly(ethylene glycol) chains
Abstract
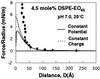
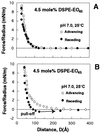
The surface force apparatus was used to measure directly the molecular forces between streptavidin and lipid bilayers displaying grafted Mr 2,000 poly(ethylene glycol) (PEG). These measurements provide direct evidence for the formation of relatively strong attractive forces between PEG and protein. At low compressive loads, the forces were repulsive, but they became attractive when the proteins were pressed into the polymer layer at higher loads. The adhesion was sufficiently robust that separation of the streptavidin and PEG uprooted anchored polymer from the supporting membrane. These interactions altered the properties of the grafted chains. After the onset of the attraction, the polymer continued to bind protein for several hours. The changes were not due to protein denaturation. These data demonstrate directly that the biological activity of PEG is not due solely to properties of simple polymers such as the excluded volume. It is also coupled to the competitive interactions between solvent and other materials such as proteins for the chain segments and to the ability of this material to adopt higher order intrachain structures.
Figures


References
-
- Harris, J. M., ed. Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications (Plenum, New York).
-
- Barenberg S A. MRS Bull. 1991;16:26–33.
-
- Elbert L, Hubbell J A. Annu Rev Mater Sci. 1996;26:365–394.
-
- Amiji M, Park K. J Biomater Sci Polym Ed. 1993;4:217–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

