Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation
- PMID: 9237989
- PMCID: PMC22934
- DOI: 10.1073/pnas.94.16.8405
Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation
Abstract
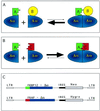
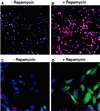
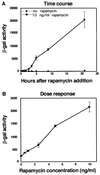
We present an approach for monitoring protein-protein interactions within intact eukaryotic cells, which should increase our understanding of the regulatory circuitry that controls the proliferation and differentiation of cells and how these processes go awry in disease states such as cancer. Chimeric proteins composed of proteins of interest fused to complementing beta-galactosidase (beta-gal) deletion mutants permit a novel analysis of protein complexes within cells. In this approach, the beta-gal activity resulting from the forced interaction of nonfunctional weakly complementing beta-gal peptides (Deltaalpha and Deltaomega) serves as a measure of the extent of interaction of the non-beta-gal portions of the chimeras. To test this application of lacZ intracistronic complementation, proteins that form a complex in the presence of rapamycin were used. These proteins, FRAP and FKBP12, were synthesized as fusion proteins with Deltaalpha and Deltaomega, respectively. Enzymatic beta-gal activity served to monitor the formation of the rapamycin-induced chimeric FRAP/FKBP12 protein complex in a time- and dose-dependent manner, as assessed by histochemical, biochemical, and fluorescence-activated cell sorting assays. This approach may prove to be a valuable adjunct to in vitro immunoprecipitation and crosslinking methods and in vivo yeast two-hybrid and fluorescence energy transfer systems. It may also allow a direct assessment of specific protein dimerization interactions in a biologically relevant context, localized in the cell compartments in which they occur, and in the milieu of competing proteins.
Figures

References
-
- Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Cell. 1993;72:309–324. - PubMed
-
- Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Science. 1995;267:1018–1021. - PubMed
-
- Schneider J W, Gu W, Zhu L, Mahdavi V, Nadal-Ginard B. Science. 1994;264:1467–1471. - PubMed
-
- Bai C, Elledge S J. Methods Enzymol. 1996;273:331–347. - PubMed
-
- Fields S, Song O. Nature (London) 1989;340:245–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

