Structural flexibility in transcription complex formation revealed by protein-DNA photocrosslinking
- PMID: 9237997
- PMCID: PMC22952
- DOI: 10.1073/pnas.94.16.8450
Structural flexibility in transcription complex formation revealed by protein-DNA photocrosslinking
Abstract
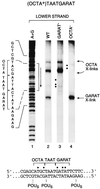
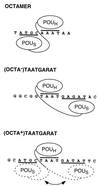
The Oct-1 POU domain binds diverse DNA-sequence elements and forms a higher-order regulatory complex with the herpes simplex virus coregulator VP16. The POU domain contains two separate DNA-binding domains joined by a flexible linker. By protein-DNA photocrosslinking we show that the relative positioning of the two POU DNA-binding domains on DNA varies depending on the nature of the DNA target. On a single VP16-responsive element, the POU domain adopts multiple conformations. To determine the structure of the Oct-1 POU domain in a multiprotein complex with VP16, we allowed VP16 to interact with previously crosslinked POU-domain-DNA complexes and found that VP16 can associate with multiple POU-domain conformations. These results reveal the dynamic potential of a DNA-binding domain in directing transcriptional regulatory complex formation.
Figures




References
-
- Herr W, Sturm R A, Clerc R G, Corcoran L M, Baltimore D, Sharp P A, Ingraham H A, Rosenfeld M G, Finney M, Ruvkun G, Horvitz H R. Genes Dev. 1988;2:1513–1516. - PubMed
-
- Klemm J D, Rould M A, Aurora R, Herr W, Pabo C O. Cell. 1994;77:21–32. - PubMed
-
- Jacobson E M, Li P, Leon-del-Rio A, Rosenfeld M G, Aggarwal A K. Genes Dev. 1997;11:198–212. - PubMed
-
- Herr W, Cleary M A. Genes Dev. 1995;9:1679–1693. - PubMed
-
- Li P, He X, Gerrero M R, Mok M, Aggarwal A, Rosenfeld M G. Genes Dev. 1993;7:2483–2496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

