Ultrafast signals in protein folding and the polypeptide contracted state
- PMID: 9238013
- PMCID: PMC23003
- DOI: 10.1073/pnas.94.16.8545
Ultrafast signals in protein folding and the polypeptide contracted state
Abstract
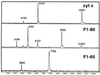
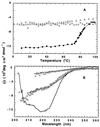
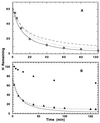
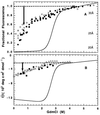
To test the significance of ultrafast protein folding signals (<<1 msec), we studied cytochrome c (Cyt c) and two Cyt c fragments with major C-terminal segments deleted. The fragments remain unfolded under all conditions and so could be used to define the unfolded baselines for protein fluorescence and circular dichroism (CD) as a function of denaturant concentration. When diluted from high to low denaturant in kinetic folding experiments, the fragments readjust to their new baseline values in a "burst phase" within the mixing dead time. The fragment burst phase reflects a contraction of the polypeptide from a more extended unfolded condition at high denaturant to a more contracted unfolded condition in the poorer, low denaturant solvent. Holo Cyt c exhibits fluorescence and CD burst phase signals that are essentially identical to the fragment signals over the whole range of final denaturant concentrations, evidently reflecting the same solvent-dependent, relatively nonspecific contraction and not the formation of a specific folding intermediate. The significance of fast folding signals in Cyt c and other proteins is discussed in relation to the hypothesis of an initial rate-limiting search-nucleation-collapse step in protein folding [Sosnick, T. R., Mayne, L. & Englander, S. W. (1996) Proteins Struct. Funct. Genet. 24, 413-426].
Figures
References
-
- Roder H, Colon W. Curr Opin Struct Biol. 1997;7:15–28. - PubMed
-
- Sosnick T R, Mayne L, Englander S W. Proteins Struct Funct Genet. 1996;24:413–426. - PubMed
-
- Sosnick T R, Mayne L, Hiller R, Englander S W. Nat Struct Biol. 1994;1:149–156. - PubMed
-
- Gross E. Methods Enzymol. 1967;11:238–255.
-
- Englander, S. W. & Englander, J. J. (1972) Methods Enzymol. 406–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

