Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase
- PMID: 9238074
- PMCID: PMC23187
- DOI: 10.1073/pnas.94.16.8895
Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase
Abstract
Protoporphyrinogen IX oxidase is the last enzyme in the common pathway of heme and chlorophyll synthesis and provides precursor for the mitochondrial and plastidic heme synthesis and the predominant chlorophyll synthesis in plastids. We cloned two different, full-length tobacco cDNA sequences by complementation of the protoporphyrin-IX-accumulating Escherichia coli hemG mutant from heme auxotrophy. The two sequences show similarity to the recently published Arabidopsis PPOX, Bacillus subtilis hemY, and to mammalian sequences encoding protoporphyrinogen IX oxidase. One cDNA sequence encodes a 548-amino acid residues protein with a putative transit sequence of 50 amino acid residues, and the second cDNA encodes a protein of 504 amino acid residues. Both deduced protein sequences share 27.2% identical amino acid residues. The first in vitro translated protoporphyrinogen IX oxidase could be translocated to plastids, and the approximately 53-kDa mature protein was detected in stroma and membrane fraction. The second enzyme was targeted to mitochondria without any detectable reduction in size. Localization of both enzymes in subcellular fractions was immunologically confirmed. Steady-state RNA analysis indicates an almost synchronous expression of both genes during tobacco plant development, greening of young seedlings, and diurnal and circadian growth. The mature plastidal and the mitochondrial isoenzyme were overexpressed in E. coli. Bacterial extracts containing the recombinant mitochondrial enzyme exhibit high protoporphyrinogen IX oxidase activity relative to control strains, whereas the plastidal enzyme could only be expressed as an inactive peptide. The data presented confirm a compartmentalized pathway of tetrapyrrole synthesis with protoporphyrinogen IX oxidase in plastids and mitochondria.
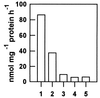
Figures






References
-
- Dailey H A, editor. Biosynthesis of Heme and Chlorophyll. New York: McGraw–Hill; 1990.
-
- Beale S I, Weinstein J D. In: Biosynthesis of Heme and Chlorophyll. Dailey H A, editor. New York: McGraw–Hill; 1990. pp. 287–391.
-
- Sano S, Granick S. J Biol Chem. 1961;236:1173–1180. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

